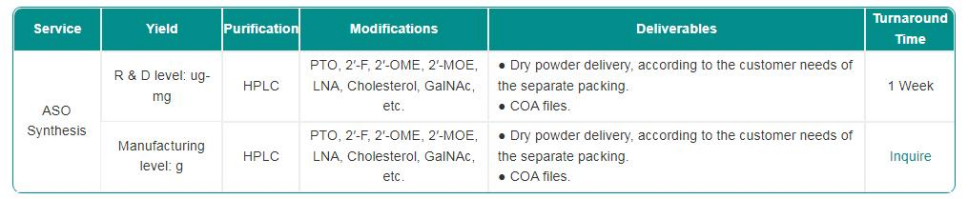
At Synbio Technologies, we recognize that high-quality ASO Synthesis is critical for clinical research and therapeutic applications. Our team ensures that every batch of antisense oligonucleotides meets rigorous standards, supporting researchers and clinicians in obtaining reliable results. Through our carefully designed processes, we integrate multiple Antisense oligo technology checkpoints to confirm purity, sequence integrity, and functionality. This attention to detail forms the foundation for safe and reproducible clinical experiments and reduces variability in downstream applications.
Rigorous Sequence Verification and Purity Assessment
One of the first steps in our ASO Synthesis workflow is verifying the nucleotide sequence. We employ precise analytical methods to confirm that the synthesized oligonucleotides match the intended design. This verification is complemented by purity assessments using high-performance techniques, ensuring minimal contamination or truncated products. By applying these Antisense oligo technology measures early in production, we can detect inconsistencies before they impact experimental outcomes. Maintaining strict control at this stage helps guarantee that clinical studies receive oligonucleotides with predictable biological activity.
Functional and Stability Testing
Beyond sequence verification, we conduct functional tests to ensure that each antisense oligo behaves as expected in relevant biological models. Stability evaluations under different storage and handling conditions are also essential. Our approach to ASO Synthesis incorporates routine monitoring of degradation patterns and shelf-life predictions. Integrating Antisense oligo technology strategies here allows us to maintain consistent performance and provide clients with oligos that are both reliable and reproducible. These steps are particularly important for clinical applications where the efficacy of therapeutic molecules can be influenced by minor structural variations.
Documentation and Batch Traceability
In clinical oligo production, maintaining detailed records is a key part of quality assurance. We implement comprehensive batch documentation to track every stage of synthesis, from raw materials to final product. Through our ASO Synthesis system, we can trace potential sources of variation and ensure regulatory compliance. The application of Antisense oligo technology extends to quality reporting and customer communication, giving our partners confidence in the safety and consistency of the antisense oligonucleotides they receive.
Conclusion
Quality control in antisense oligo production is not limited to a single checkpoint but requires an integrated approach. At Synbio Technologies, we combine ASO Synthesis expertise, Antisense oligo technology techniques, and rigorous documentation to support clinical research and therapeutic development. By focusing on sequence accuracy, purity, functional stability, and traceability, we provide oligonucleotides that meet the high standards required for clinical use. Our commitment ensures that clients receive reliable, high-quality products that advance both research and potential treatments in a controlled and reproducible manner.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























