General Introduction of ELISA Kit Development
As a classical immunodetection method, ELISA (Enzyme-Linked Immunosorbent Assay) relies on specific antigen–antibody interactions to enable accurate quantitative analysis of target proteins. It is widely applied in disease biomarker detection, drug development, immune response studies, and quality control.
Synbio Technologies provides end-to-end ELISA development services, from standard assay formats to fully customized, application-specific solutions. We ensure high performance in sensitivity, specificity, reproducibility, and stability. Beyond ELISA, our IVD reagent development services support both research and clinical translation through efficient, professional collaboration.
Highlights
-
![]()
- One-Stop Solution
From antigen design to large-scale production.
-
![]()
- Broad Target Coverage
Comprehensive detection for small molecules, peptides, and proteins.
-
![]()
- Strong Antibody Development Capability
Leveraging both single B-cell and hybridoma platforms.
-
![]()
- Rigorous QC System
Full-process validation ensures reliable and consistent results.
Service Details
|
Service Stage
|
Service Content
|
Timeline
|
|
Project Consultation
|
Determine ELISA development type and plan based on detection targets and specific requirements
|
Quote
|
|
Antibody Preparation (Optional)
|
- Antigen Preparation
- Animal Immunization
- B Cell Screening
- High-Throughput Expression & Validation
|
Quote
|
|
Antigen/Antibody Labeling & Pairing
|
- Antigen/Antibody Labeling
- Antigen/Antibody Pairing
|
2-3 weeks
|
|
Proof of Concept (POC)
|
- Preliminary ELISA kit detection optimization
- Preliminary standard curve establishment
- Preliminary recovery rate evaluation
|
10-12 weeks
|
|
Method Establishment
|
- Kit optimization: reaction conditions and other indicators
- Standard curve confirmation (positive and negative samples)
- Recovery rate confirmation (positive and negative samples)
|
|
Method Validation
|
- Sample testing
- Sensitivity testing
- Precision testing
- Stability testing
|
Workflow
Deliverables
-
2-5 ELISA kits (including all components and user manual)
-
Performance Test Report (Certificate of Analysis, CoA)
Case Study
1.Anti-target A Rabbit mAb Pairs
-
Deliver the positive rabbit mAb clones by FACS sorting methods.
-
The affinity and sensitivity of one clone 5F7 is better than the commercialized antibody (from Abcam) and rabbit pAb.
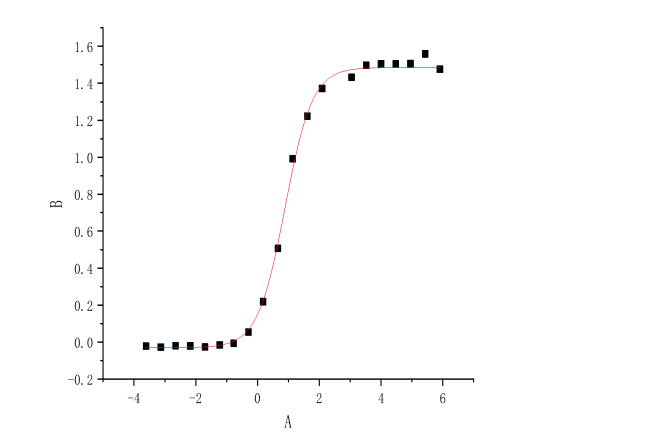
The recombinant monoclonal antibody recognized protein A (KD ≈ 3.19E-10M, affinity pM)
2.Anti-target B ELISA Kit
-
Deliver the 15 positive rabbit mAb clones by two rounds of B cell cloning method.
-
The affinity and sensitivity of three clones 1H7, 2D5, 2G8 could be reached out for ELISA pairing testing, which were better than mouse mAb clones.
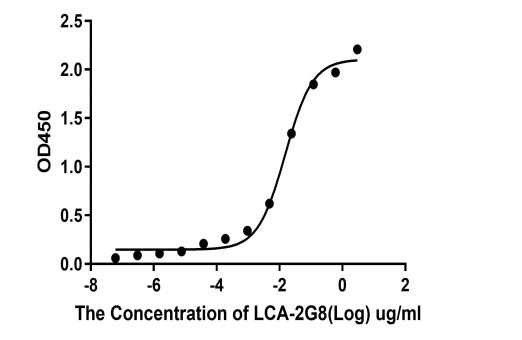
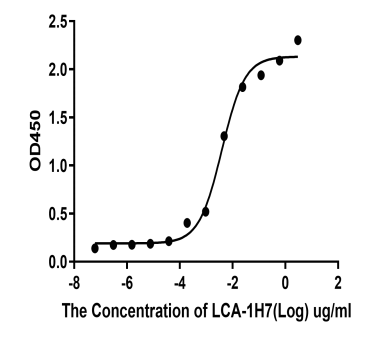
Recombinant monoclonal antibody recognizes Protein B (KD ~2.27E-10 M, pM affinity)
FAQs
Yes. We can proceed directly to pairing validation and kit assembly if you have validated a capture and detection antibody, which shortens the timeline. Please share antibody details (species, subtype, purity, titer, etc.) for evaluation.
Typically 10-12 weeks for standard development with existing antibodies. Timelines may extend for novel targets or custom requirements. We’ll collaborate with you to define a clear schedule upfront.
Get in Touch with Us
Related Services
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products