CRISPR/Cas9 is a powerful gene editing technology that enables precise gene knockout, insertion, or correction.
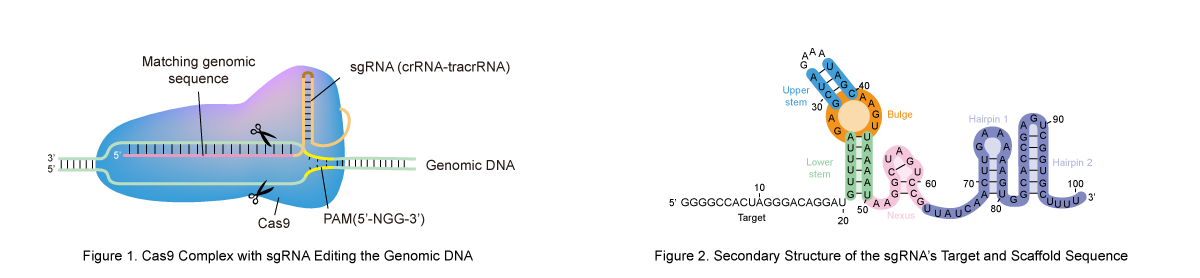
In the CRISPR/Cas9 system, single-guide RNA (sgRNA) acts as the guiding molecule that directs the Cas9 nuclease to a specific DNA sequence. It combines two functional parts: a CRISPR RNA (crRNA) matching the target DNA and a trans-activating crRNA (tracrRNA) that binds Cas9. Once properly guided, Cas9 causes a double-strand break at the target location, initiating the gene editing process.
Thus, sgRNA design is essential for improving CRISPR experiment efficiency, reproducibility, and editing precision. What are the influence factors and how to optimize?
PAM Sequence Requirements
The Protospacer Adjacent Motif (PAM) is a short DNA sequence located immediately downstream of the target DNA sequence. Without a valid PAM, the Cas9 protein cannot undergo the conformational change required to "unzip" the DNA double helix for the sgRNA sequence to bind.
The most commonly used nuclease, Streptococcus pyogenes Cas9 (SpCas9), recognizes a 5'-NGG-3' PAM, where 'N' can be any nucleotide. Different Cas9 variants exhibit distinct PAM preferences. For example:
-
SaCas9: Recognizes 5'-NNGRRT-3', offering a larger but more specific footprint.
-
Cas12a: Preferentially targets T-rich regions with a 5'-TTTV-3' PAM, providing flexibility for genomes that are difficult to edit with standard SpCas9.
Choosing the right PAM is the first rule to design sgRNA successfully. If the PAM interaction is weak or the sequence is suboptimal, the residence time of the Cas9 on the DNA is reduced, leading to a significant drop in editing efficiency.
sgRNA Sequence Design
The composition of an sgRNA sequence is the primary driver of efficiency.
1. Target Sequence Length
The ideal sgRNA sequence used with SpCas9 consists of a 17-23 nucleotide spacer that is complementary to the target DNA. This length has been optimized to balance targeting specificity and cleavage efficiency.
Shorter sequences reduce specificity, while longer sequences may increase off-target risks. Maintaining the correct sgRNA sequence length is a fundamental step in sgRNA design.
2. GC Content
The GC content of the sgRNA sequence significantly impacts its binding energy, as GC pairs are more stable than AT pairs. The ideal GC content generally ranges between 40% and 60%.
-
High GC Content (>70%): May result in rigid secondary structures or increased off-target activity.
-
Low GC Content (<30%): Leads to weak RNA-DNA duplex stability, preventing the Cas9 complex from remaining on the target long enough for double-strand break induction.
3. Sequences to Avoid
Proper CRISPR sgRNA design must avoid specific inhibitory motifs. For instance, consecutive Guanines (e.g., GGGGG) can cause the sgRNA CRISPR complex to fold incorrectly or aggregate, thereby abolishing functional activity.
Secondary Structure of sgRNA
The sgRNA is more than just a linear sequence; it is a structural scaffold. It consists of a spacer region (for targeting) and a scaffold region (for Cas9 binding). The scaffold must form specific stem-loops and hairpins to fit into the binding groove of the Cas9 protein.
If the spacer region is complementary to the scaffold region, the sgRNA may undergo internal misfolding. This "self-binding" creates hairpins that shield the targeting sequence, making it unavailable for DNA hybridization.
To reduce unfavorable secondary structures, some RNA folding prediction tools can be used during the design sgRNA processes to minimize internal base pairing while preserving scaffold integrity.
Off-Target Activity
A significant challenge in CRISPR sgRNA design is ensuring that the nuclease only cuts the intended site. Off-target activity occurs when the sgRNA guides Cas9 to a genomic location with a similar sequence but one or more mismatches.
1. Mismatches and the "Seed Region"
The 8-12 nucleotides closest to the PAM are known as the "seed region." This area is extremely sensitive to mismatches. Even a single base-pair discrepancy in the seed region can completely eliminate Cas9 activity.
However, mismatches further from the PAM are often tolerated by the enzyme, leading to unintended mutations elsewhere in the genome.
2. Balancing Specificity and Activity
The goal of high-quality sgRNA design is to maximize on-target activity while minimizing potential off-target site risks. Researchers utilize tools to scan the entire genome for potential mismatches, ranking candidates based on their likelihood of being cleaved.
Cellular Context
The physical environment of the DNA is as important as the sgRNA sequence itself. In eukaryotic cells, DNA is wrapped around histones to form chromatin.
1. Chromatin State and Accessibility
Active gene regions (euchromatin) are physically accessible and generally show high editing efficiency. In contrast, highly condensed regions (heterochromatin) can physically block the Cas9 protein from accessing the DNA.[1]
Therefore, an sgRNA CRISPR complex that performs well in a biochemical assay may fail in a cell line where the target site is buried within heterochromatin.
2. Cell Type Variations
Different cell types have varying DNA repair mechanisms and intracellular environments. Factors such as endogenous nuclease levels and temperature can influence how the sgRNA sequence interacts with the target, necessitating cell-specific optimization during the R&D phase.
Delivery Methods
How the sgRNA CRISPR components are introduced into the cell significantly impacts the final efficiency. Common delivery approaches include plasmid and viral vectors. Plasmid delivery enables sustained sgRNA expression but may introduce variability due to transcription efficiency. Viral vectors provide high delivery efficiency in difficult-to-transfect cells but may raise concerns related to integration or immune responses.
Each delivery method influences sgRNA stability, expression level, and timing, all of which interact with sgRNA design.
How to Optimize sgRNA Design?
To achieve maximal performance in CRISPR experiments, researchers are increasingly moving toward synthetic sgRNA solutions.
1. The Power of Chemical Modifications
Synthetic production allows for the introduction of chemical modifications that are impossible via biological expression. To protect the sgRNA sequence from exonucleases and reduce innate immune responses. For example:
-
2'-O-Methyl (2'-OMe): Enhances RNA stability and binding affinity.
-
Phosphorothioate linkages: Provides a "shield" against enzymatic degradation at the 5' and 3' ends.
2. Elevate Your Research with Synbio Technologies
Synbio Technologies offers two sgRNA synthesis services, including chemical synthesis and in vitro transcription. Our platform provides:
-
High-Purity Synthesis: Provide 100% accurate sgRNA sequences. These sgRNAs have high stability and low toxicity, ensuring efficient gene editing.
-
Custom Chemical Modifications: Modification options (2’-OMe and Phosphorothioate) to enhance stability in vivo and in vitro.
-
High-Purity Synthesis: HPLC purification offers batch-to-batch consistency.
Whether you are performing high-throughput drug screening or developing the next generation of vaccines, the reliability of your sgRNA CRISPR components is non-negotiable. Contact Synbio Technologies now for ready-to-use sgRNA products.
References
[1] Factors affecting the cleavage efficiency of the CRISPR-Cas9 system. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC10911232/ (Accessed: 31 December 2025)
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























