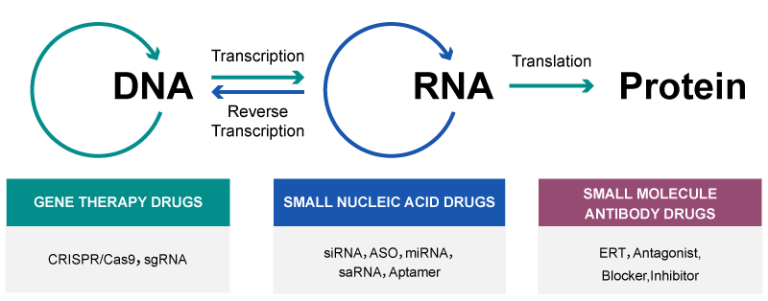
The evolution of modern medicine has moved from small-molecule drugs toward nucleic acid-based therapeutics, marking a shift from protein-level intervention to direct regulation at the genetic information level.
RNA therapeutics represent this transformation by enabling precise control over gene expression rather than protein activity. Compared with DNA-based approaches, RNA drug offers higher safety due to its non-permanent nature, while RNA medicine is more versatile than protein drugs because it can theoretically target any disease-related gene.
Currently, RNA therapies encompass various molecules, including mRNA, siRNA, miRNA, ASO, saRNA, and Aptamers, offering new avenues for drug development.
Major Types of RNA Therapeutics
RNA therapy is a type of treatment that uses RNA molecules to regulate gene expression and protein production within cells. It works at the genetic level by delivering specific RNA sequences to either produce therapeutic proteins or silence disease-causing genes.
The field of RNA therapy is diverse, encompassing several molecules, such as:
1. mRNA
These molecules serve as the direct templates for protein synthesis. Once they enter the cytoplasm, they are translated by the cell’s own machinery into functional proteins.
It makes them ideal for vaccines, protein replacement therapies, and providing the instructions for gene editing tools.
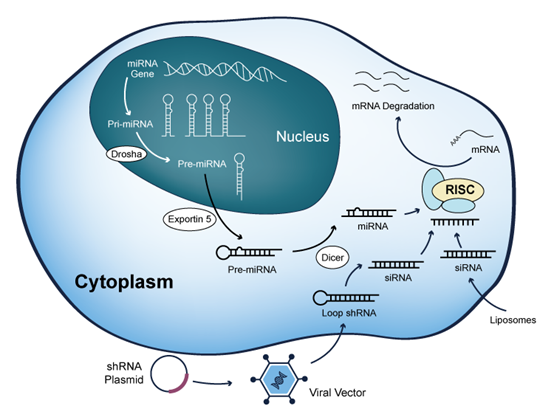
2. RNA Interference (RNAi)
RNA interference is a natural cellular mechanism used to regulate gene expression.
-
siRNA (Small Interfering RNA): These are short, double-stranded fragments that guide the RNA-induced silencing complex (RISC) to cleave complementary mRNA strands, effectively silencing disease-causing genes before they can produce harmful proteins.
-
miRNA (microRNA): These are endogenous single-stranded molecules that regulate broad gene networks by either inhibiting the translation of target mRNA or promoting its degradation.
3. Antisense Oligonucleotides (ASO)
ASOs are single-stranded nucleic acids designed to bind complementary mRNA sequences. They either trigger mRNA degradation or modulate their splicing patterns.
ASO-based RNA drugs have already demonstrated clinical success in genetic diseases such as spinal muscular atrophy.
4. Self-amplifying RNA (saRNA)
Derived from viral vectors, saRNA includes the machinery for its own replication. This allows the molecule to amplify itself once inside the cell, providing more sustained protein expression or immune stimulation at significantly lower initial doses compared to conventional mRNA.
5. Aptamers
Aptamers are synthesized in vitro. Often described as "chemical antibodies," these are structured RNA sequences that fold into unique three-dimensional shapes. This allows them to bind directly to specific protein targets with high affinity, physically blocking their biological activity. They can also serve as targeting ligands for drug delivery.
How RNA Therapeutics Solve Traditional Drug Development Hurdles?
The rise of RNA therapeutics is driven by their ability to overcome the limitations of antibody drugs.
1. Overcoming "Undruggable" Targets
Traditional drug discovery is often stymied by the fact that only about 0.05% of the human genome is currently targeted by existing drugs. Approximately 85% of proteins lack the defined "pockets" or clefts required for small molecule binding.[1]
RNA drugs bypass this limitation entirely. Instead of attempting to bind to a complex protein structure, RNA-based interventions target the mRNA sequence that encodes that protein through simple nucleotide base-pairing.
2. Rapid Development and Flexibility
RNA therapy relies on sequence design rather than complex protein engineering. A unified production platform can be rapidly adapted to different targets by changing the nucleotide sequence.
This was vividly demonstrated by the rapid R&D and deployment of mRNA vaccines during the COVID-19 pandemic.
3. High Targeting Specificity
The precision of RNA therapy is rooted in the fundamental laws of biology: complementary base pairing.
By designing sequences that only match a specific mRNA transcript, researchers can achieve high specificity with minimal off-target effects. This high efficiency within the cell environment often allows for lower dosing regimens compared to systemic protein therapies.
4. Standardized Manufacturing Advantages
Unlike protein drugs that require complex biological fermentation in living cells, a process prone to batch-to-batch variation, RNA medicine can be synthesized through cell-free, in vitro processes. This allows for highly standardized, scalable, and reproducible manufacturing, streamlining the path from the lab to the clinic.
Critical Delivery Systems
The primary challenge for any RNA drug is its inherent instability and the difficulty of crossing the cellular membrane. To solve this, two major delivery technologies are:
1. Lipid Nanoparticles (LNP)
LNPs act as a protective "envelope" for RNA molecules, shielding them from enzymatic degradation in the bloodstream. They are the cornerstone of current mRNA vaccine technology.
Ongoing research focuses on optimizing LNP compositions to move beyond the liver and achieve specific targeting of other tissues, such as the lungs or spleen.
2. GalNAc Conjugation
This technology involves chemically linking the RNA molecule directly to N-acetylgalactosamine (GalNAc) sugars. These sugars bind with high affinity to asialoglycoprotein receptors (ASGPR) expressed almost exclusively on hepatocytes.
This has revolutionized RNA therapy for liver-related diseases, allowing for highly efficient, subcutaneous administration with prolonged duration of action.
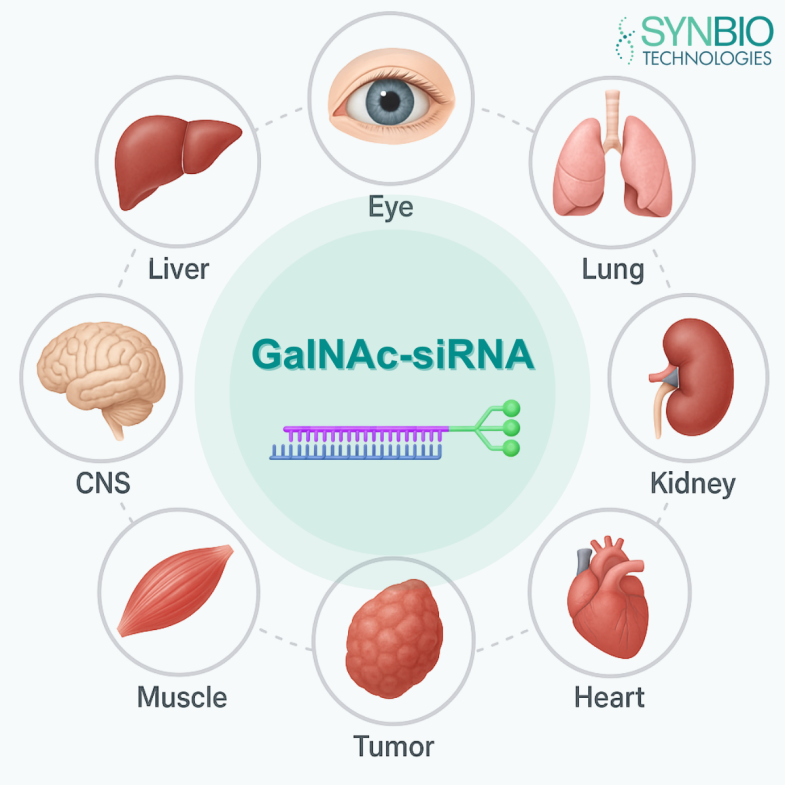
Diverse Uses of RNA Medicine
The versatility of RNA therapeutics is reflected in their broad clinical applications across various medical disciplines.
-
Infectious Diseases: Enable rapid vaccine development and antiviral strategies, such as the success of COVID-19 vaccines.
-
Genetic and Rare Diseases: RNA-based drugs have transformed the prognosis for previously untreatable conditions, such as the use of ASOs for spinal muscular atrophy.
-
Oncology: Include cancer vaccines and gene-silencing approaches targeting oncogenic drivers.
-
Metabolic and Chronic Conditions: RNA interventions are being developed for prevalent conditions like hypercholesterolemia, chronic Hepatitis B (HBV), and more.
RNA therapeutics are being a versatile platform for future medicine.
Professional RNA Synthesis from Synbio Technologies
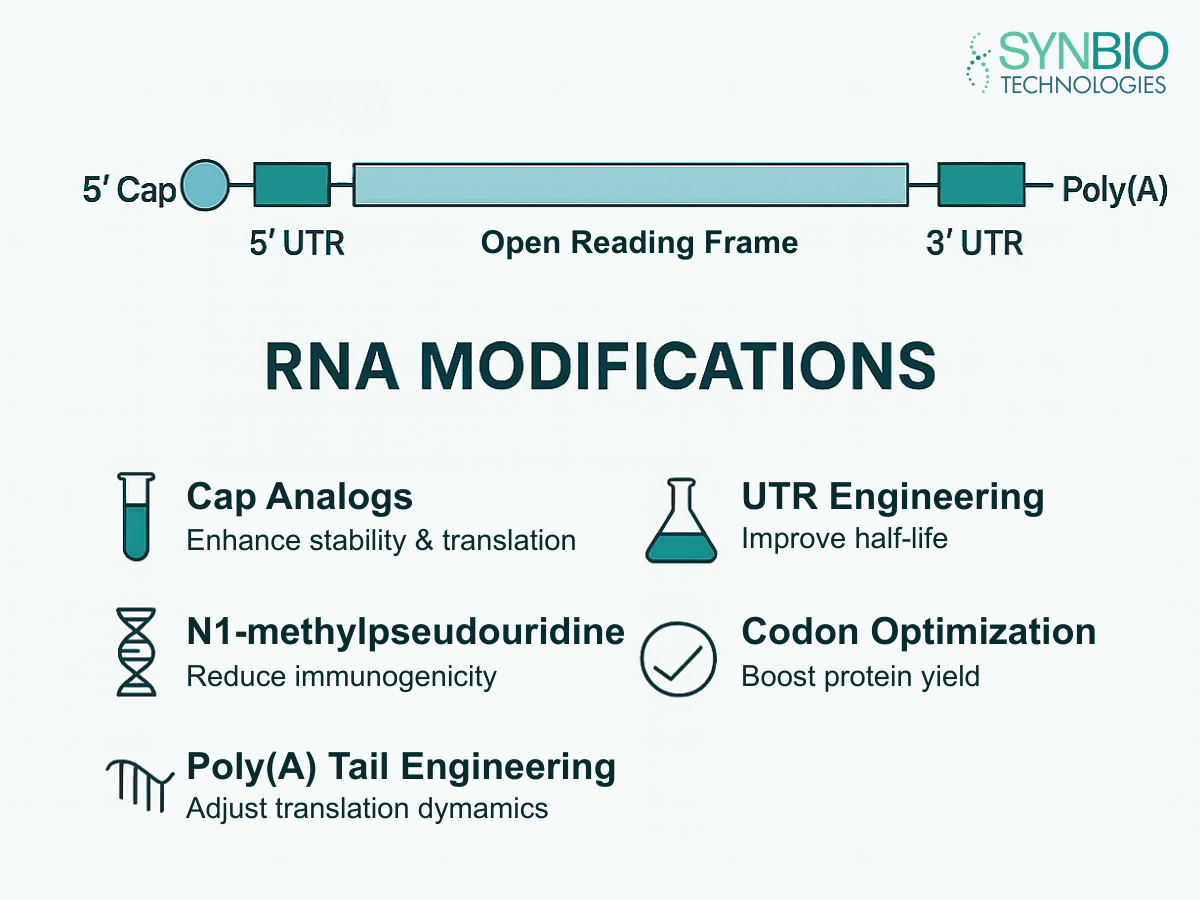
As the demand for high-quality RNA medicine research grows, the need for precision synthesis services becomes paramount. Synbio Technologies provides comprehensive, professional RNA synthesis services for researchers.
Our platform offers a wide array of chemical modifications to enhance the stability and potency of your RNA drug candidates. With a focus on high purity, verified sequences, and flexible scales ranging from pilot studies to large-scale production, Synbio Technologies empowers R&D managers and scientists in gene therapy, IVD, drug discovery, etc.
Whether you are exploring RNAi mechanisms or developing novel delivery systems, our expert team ensures that your therapeutic sequences are synthesized with the highest technical precision to accelerate your clinical success.
Contact Synbio Technologies now for more details.
References
[1] RNA-based therapeutics: an overview and prospectus. Available at: https://www.nature.com/articles/s41419-022-05075-2#Sec1 (Accessed: 31 December 2025)
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























