Nanobodies are single-domain antibody fragments derived from the heavy-chain-only antibodies naturally found in camelids. Owing to their small size, high stability, and strong binding affinity, nanobodies have emerged as versatile tools in diagnostics, therapeutics, and structural biology.
But how is a high-affinity, highly specific nanobody actually developed?
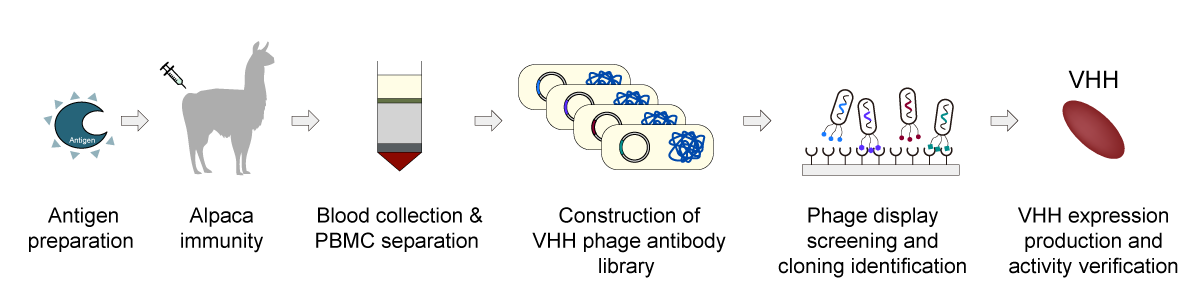
This case study demonstrates how a proven alpaca immunization and phage display workflow rapidly generated multiple validated nanobody candidates against a peptide target (B-N), delivering results within months rather than years.
End-to-End Workflow Overview
Objective:
Generate high-affinity nanobodies targeting peptide B-N using a robust and scalable discovery platform.
Approach:
Alpaca immunization combined with high-quality phage display library construction and stringent screening.
Step 1: Alpaca Immunization & Library Construction
Immunization strategy: Healthy adult alpacas were immunized over multiple rounds, with serum titers monitored by ELISA to confirm a strong and sustained immune response.
Library generation: Peripheral blood was collected after the final boost. Lymphocytes were isolated, total RNA extracted, and cDNA synthesized. Nanobody (VHH) genes were specifically amplified and assembled into a high-quality phage display library for downstream screening.

B-N the second round of PCR amplification electrophoresis
Quality Assessment: The primary phage display library reached a capacity of 1.46×109, ensuring broad sequence diversity. Random clone sequencing showed a 93.3% correctness rate, yielding an effective library size of 1.36×109, providing a strong foundation for downstream selection.
Step 2: Phage Display Screening
Affinity panning: Using the immobilized target protein (B-N-BSA) as bait, the library underwent multiple rounds of selective enrichment. Each round progressively retained phage particles capable of binding the target with higher specificity.
Enrichment performance: After two rounds, the library showed clear enrichment. ELISA readouts (OD450) against the target were substantially above background, indicating successful recovery of target-specific clones.
Step 3: Monoclonal Screening & Identification
High-throughput prescreening: A total of 192 clones from the enriched pool were expressed and evaluated using a high-throughput ELISA format.
Specificity verification:
To eliminate nonspecific binders, all positive hits were tested against controls including BSA and blank wells.
Outcome: Eight nanobody candidates exhibited strong binding to B-N-BSA with no detectable cross-reactivity. Several clones showed OD450 values above3.0, reflecting excellent binding performance.
Project Summary
The platform delivered multiple validated candidate binders within just a few months, demonstrating strong operational efficiency.
A high-diversity library combined with a rigorous selection workflow ensured that the resulting nanobodies showed promising affinity and specificity.

This case demonstrates a robust and efficient nanobody discovery pipeline built on alpaca immunization and phage display. Yet obtaining a strong monomeric nanobody is often only the beginning. The next challenge is transforming these “small but powerful” binders into precise molecular tools capable of tackling complex biomedical problems.
Frontier Trends: Emerging Directions in Nanobody Innovation
Nanobody development is evolving beyond traditional antibody replacement toward precision-engineered, multifunctional biologics.
1. Structure-Guided Engineering & Combination (“Cocktail”) Therapies
On November 19, 2025, a study in Science Translational Medicine reported a major breakthrough in nanobody-based antiviral therapeutics[1]. Using a sequential heterologous immunization strategy in alpacas, researchers identified nanobodies targeting the emerging tick-borne virus SFTSV.
Through structural analysis, the team rationally designed a two-nanobody cocktail (Nb261 + Nb318), each recognizing a distinct epitope. This combination successfully protected mice and ferrets from lethal SFTSV challenge—representing the first systematic demonstration of a mixed-nanobody therapy in a large animal model.
The work highlights both a promising drug candidate for SFTSV and the value of alpaca-derived nanobodies as a rapid countermeasure for emerging pathogens.
2. Multi-Target and Multi-Functional Nanobody Architectures
Nanobodies are highly modular, making it straightforward to assemble constructs targeting multiple antigens.
Recent examples include: Dual-specific nanobodies developed by CAS researchers, designed to simultaneously block tumor immune-suppression signals and angiogenesis pathways, achieving strong anti-tumor and anti-metastatic effects in animal models[2].
3. AI-Driven Design: A New Era of Computational Nanobody Engineering
While traditional nanobody discovery relies on immunization and library screening, AI is now transforming the landscape. Machine-learning models can predict antigen–nanobody interactions, optimize affinity and stability, and guidede novo design. This accelerates discovery, reduces experimental cycles, and raises the overall precision of nanobody engineering[3].
Conclusion & Outlook
With ongoing progress in structural design, multi-functional integration, and next-generation applications including cell-based therapies, nanobodies are positioned to play an increasingly influential role in precision diagnostics, targeted therapeutics, and advanced biotechnology.
This case study demonstrates how a reliable discovery workflow can translate biological diversity into powerful molecular tools ready for real-world biomedical challenges.
References
[1]Wu X, Zhu L, Liang S, et al. A rationally designed cocktail of nanobodies elicited by heterologous vaccination confers protection against SFTSV in preclinical models. Sci Transl Med. 2025 Nov 19;17(825):eady9025.
[2]Zhang L, Lin Y, Hu L, et al. Transient intracellular expression of PD-L1 and VEGFR2 bispecific nanobody in cancer cells inspires long-term T cell activation and infiltration to combat tumor and inhibit cancer metastasis. Mol Cancer. 2025 Apr 19;24(1):119.
[3]Liu, J., Wu, L., Xie, A. et al. Unveiling the new chapter in nanobody engineering: advances in traditional construction and AI-driven optimization. J Nanobiotechnol 23, 87 (2025).
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























