In the fields of gene therapy and biomedical research, choosing the right delivery vehicle is as critical as the genetic payload itself.
Viral vectors, particularly Adeno-associated virus (AAV) and Lentivirus, are the mainstream in modern biotechnology, enabling precise gene delivery for everything from basic research to life-saving clinical treatments.
Understanding the nuances of AAV vs lentivirus is essential for optimizing experimental safety, efficiency, and long-term therapeutic success.
About Adeno-associated Virus (AAV)
Adeno-associated virus is a small, non-enveloped virus belonging to the pendoparvovirus genus. Structurally, it consists of a simple icosahedral protein capsid protecting a single-stranded DNA (ssDNA) genome of approximately 4.7 kb.
One of the defining features of AAV vectors is their non-pathogenic nature; they are not known to cause any human disease, making them a top-tier choice for clinical safety.
Gene delivery mechanism:
The gene delivery mechanism of the AAV virus vs lentivirus is fundamentally different. AAV functions as a non-integrating vector. Upon entering the target cell, the ssDNA genome is converted into double-stranded DNA, which primarily persists in the cell nucleus as stable, circularized episomes.
Because the DNA remains separate from the host genome, the risk of insertional mutagenesis, where a virus accidentally disrupts a vital gene or activates an oncogene, is significantly minimized.
Advantages of AAV
-
Low Immunogenicity: AAV rarely triggers an immune response compared to other viral systems.
-
Long-term Expression: In non-dividing cells, AAV can provide stable transgene expression for years.
-
Tissue Specificity: By utilizing different AAV vectors and their various serotypes, researchers can achieve highly targeted delivery to specific organs.
Limitations
The primary drawback of AAV versus lentivirus is the packaging capacity. With a limit of < 4.5 kb, AAV cannot accommodate large genes or complex regulatory elements. Additionally, while the safety profile is excellent, the onset of expression can be slower compared to other methods because the ssDNA must first be converted into a functional template.
About Lentivirus
Lentivirus is a genus of retroviruses characterized by a complex, enveloped structure. A conical core contains two copies of positive-sense single-stranded (ssRNA) genome and essential enzymes (reverse transcriptase, integrase, protease), surrounded by a capsid, matrix, and outer lipid envelope studded with glycoproteins for cell entry.
Gene delivery mechanism:
The hallmark mechanism of a lentiviral vector vs AAV is its integration. After entering the cell, the viral RNA is reverse-transcribed into dsDNA, which is then permanently integrated into the host cell's genome. This ensures that when the cell divides, the transgene is passed on to all daughter cells, ideal for creating stable cell lines and permanent genetic modifications.
Advantages of Lentivirus
-
High Transduction Efficiency: Lentiviruses are incredibly efficient at delivering genes into a wide variety of cell types.
-
Large Packaging Capacity: Capable of carrying 6 kb of genetic material, they are ideal for large genes that AAV cannot handle.
-
Permanent Expression: Integration ensures the gene is never lost during cell division, which is critical for virus packaging aimed at hematopoietic or stem cell therapies.
Limitations
The major concern in the AAV vs lentivirus debate regarding lentivirus is the potential for insertional mutagenesis. While modern technology has drastically improved safety by preventing the virus from replicating, the risk of disturbing the host genome remains higher than with episomal AAV.
Clinical Applications: CAR-T Therapy
Lentivirus has found its famous application in CAR-T cell therapy. By using virus packaging to insert Chimeric Antigen Receptors (CARs) into a patient’s T-cells, scientists can "program" the immune system to recognize and destroy cancer cells. The permanent integration is vital here, as the modified T-cells must expand and maintain their cancer-fighting ability throughout the patient's body.
AAV vs Lentivirus: Key Differences
To simplify the choice between AAV versus lentivirus, the following table summarizes the technical specifications and performance metrics essential for your research.
|
Feature |
AAV |
Lentivirus |
|---|---|---|
|
Viral Genome |
ssDNA |
ssRNA |
|
Integration |
Non-integrating (primarily episomal) |
Highly integrating (into host genome) |
|
Expression Onset |
Slow (2-3 weeks) |
Rapid (2-4 days) |
|
Expression Duration |
Long-term in non-dividing cells |
Permanent (passed to daughter cells) |
|
Infected Cells |
Dividing and Non-dividing |
Dividing and Non-dividing |
|
Expression Abundance |
High level in vitro |
High level |
|
Titer |
Maximum 1×1012-13VG/ml |
Maximum 1×109TU/ml |
|
Packaging Capacity |
<4.5kb |
<6kb |
|
Immunogenicity |
Very Low |
Moderate |
|
Transfection Efficiency |
High |
High |
Choosing the Right Virus Packaging Vector
Deciding between the AAV virus vs lentivirus depends entirely on the biological context of your project. Here is how to navigate the decision:
1. "I am performing in vivo experiments."
If you are injecting into a live animal model, AAV vectors are generally the superior choice. Their low immunogenicity and high tissue diffusion allow for systemic or localized delivery with minimal inflammation.
By selecting a specific serotype, you can target the brain, liver, or heart with surgical precision.
2. "I need in vitro cellular modification."
For constructing stable cell lines or performing CRISPR screens in the lab, lentivirus is the winner. The integration of the transgene ensures that your modification stays put, even as the cells replicate rapidly in culture.
3. "My gene is very large."
If your sequence (including the promoters, enhancers, and insulators) exceeds 5 kb, AAV is physically unable to package it. In the AAV vs lentivirus capacity battle, lentivirus wins, making it necessary for large genes.
4. "I am focused on clinical translation and safety."
While both are used clinically, AAV is often preferred for direct-to-patient gene therapy due to its non-integrating nature and non-pathogenic origin.
However, for "ex vivo" therapies, where cells are removed from the patient, modified, and then returned (like CAR-T), lentivirus remains the standard.
Searching for Premier Virus Packaging Services
When your research demands precision, Synbio Technologies provides industry-leading virus packaging solutions.
Synbio Technologies offers a comprehensive range of viral packaging services, including lentiviruses, adenoviruses, and adeno-associated viruses (AAV). Our platform covers the entire workflow, from gene synthesis to viral vector construction, packaging, and purification.
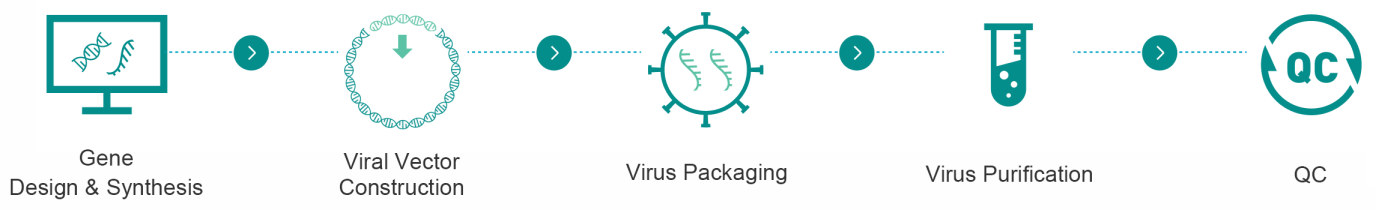
Conclusion
Choosing between AAV and lentivirus depends on your gene's size, expression needs, etc. AAV excels in safe, in vivo tissue targeting, while lentivirus is ideal for stable, large-gene integration.
For more information about virus packaging services, please contact Synbio Technologies.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























