Therapeutic antibodies have revolutionized modern medicine since the first monoclonal antibody was approved by the US Food and Drug Administration (FDA) in 1986. With their high affinity, specificity, low immunogenicity, and ability to target a multitude of biological molecules, this class of drug has become highly sought-after for its therapeutic potential. So far, the FDA has approved nearly 100 therapeutic antibody drugs.
What is the Size of the Therapeutic Antibody Drugs Market?
The therapeutic antibody drug market is rising rapidly, with the global value estimated to exceed $300 billion by 2025. With research and development of a new generation of these antibodies increasingly gaining attention from around the world, many distinct technologies are being employed in their production for varying applications. In just 5 years, this field has skyrocketed - already surpassing traditional pharmaceuticals as 2018's best-selling drug on an international scale.
Therapeutic Antibody Development | Technologies
What is Hybridoma Monoclonal Antibody Technology?
Hybridoma monoclonal antibody technology provides a powerful tool to identify and isolate specific antibodies. By immunizing mice with an antigen, spleen cells are then fused with myeloma cell lines using polyethylene glycol to create hybridomas that produce monoclonal antibodies. This technique can also harvest these specialized molecules through inoculating tumor cells into mouse abdomens for continued production of targeted immune responses.
What is Phage Display Technology?
Phage display technology is a powerful tool for studying antigen-antibody reactions, diagnostics, and vaccine development. Using gene recombination techniques, the genes of target proteins are fused with bacteriophage capsid proteins to create fusion proteins that coat the phages' surface. These customized phages can then be used to infect bacteria which, in turn, allows us to identify antibody specificities while also gathering insight into reaction mechanisms between antigens and antibodies.
What is Single B cell Technology?
Single B cell technology is revolutionizing the production of monoclonal antibodies, by offering immense genetic diversity and unparalleled efficiency. This emerging technique enables rapid preparation of antigen-specific B cells from animal tissue or blood by amplifying IgG light chain and heavy chain variable region genes via single cell PCR. Single B cell technology has become an essential strategy in quickly developing antibodies against viral infectious diseases and provide a powerful new approach to combat such medical challenges.
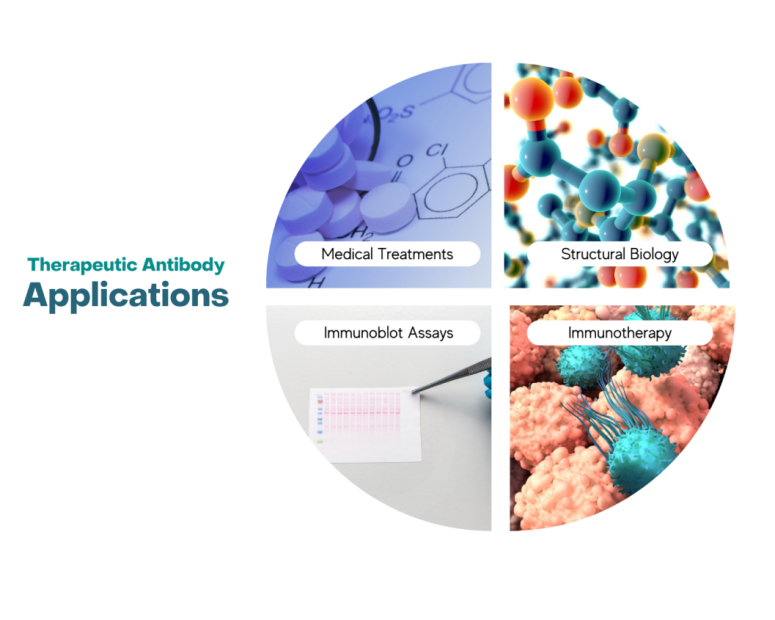
Therapeutic Antibody Development | Applications
Cancer Treatment: Monoclonal antibodies (mAbs) are designed to target specific antigens on cancer cells, leading to their destruction. Examples include trastuzumab (Herceptin) for HER2-positive breast cancer and rituximab (Rituxan) for non-Hodgkin lymphoma.
Autoimmune Diseases: Therapeutic antibodies are used to modulate immune responses in conditions such as rheumatoid arthritis, lupus, and multiple sclerosis. Examples include adalimumab (Humira) and abatacept (Orencia), which target specific immune pathways.
Infectious Diseases: Clinical trials of anti-lymphocyte monoclonal antibodies (SALM) for the treatment of malignant lymphoma have shown significant efficacy against non-Hodgkin's lymphoma. Additionally, studies have used monoclonal antibodies for epidemic encephalitis B in clinical treatment, successfully establishing a monkey model for Japanese encephalitis and conducting treatment experiments.
Immunoblot Biology
Protein Detection: Therapeutic antibodies can be used to specifically detect target proteins in complex samples, helping to confirm the presence and expression levels of therapeutic candidates or biomarkers.
Researching Protein Function and Interactions: By using immunoblotting techniques, the binding of antibodies to proteins can be observed, allowing for the inference of the protein's function, localization, and interactions with other molecules. This is of great importance for understanding disease mechanisms and developing new therapeutic strategies.
Characterization of Antibody Specificity: Immunoblotting can be used to validate the specificity of therapeutic antibodies by confirming their ability to bind only to the intended target protein and not to similar proteins.
Immunotherapy
Targeted Delivery of Cytotoxic Agents: Some therapeutic antibodies are conjugated to cytotoxic drugs or radioisotopes, allowing for targeted delivery to cancer cells while minimizing damage to healthy tissue.
Treatment of Autoimmune Diseases: Therapeutic antibodies can neutralize specific pro-inflammatory cytokines or target immune cells, helping to modulate the immune response in conditions like rheumatoid arthritis, lupus, and multiple sclerosis.
Treatment of Allergic Diseases: Specific immunotherapy is a symptomatic treatment method targeting the pathogen, which alters the natural course of allergic diseases by modulating antigen-presenting cell functions, inducing specific regulatory T cells, and promoting antibody class switching, among other mechanisms. For example, in the treatment of atopic dermatitis (AD), allergen-specific immunotherapy (ASIT) has been proven to be effective.
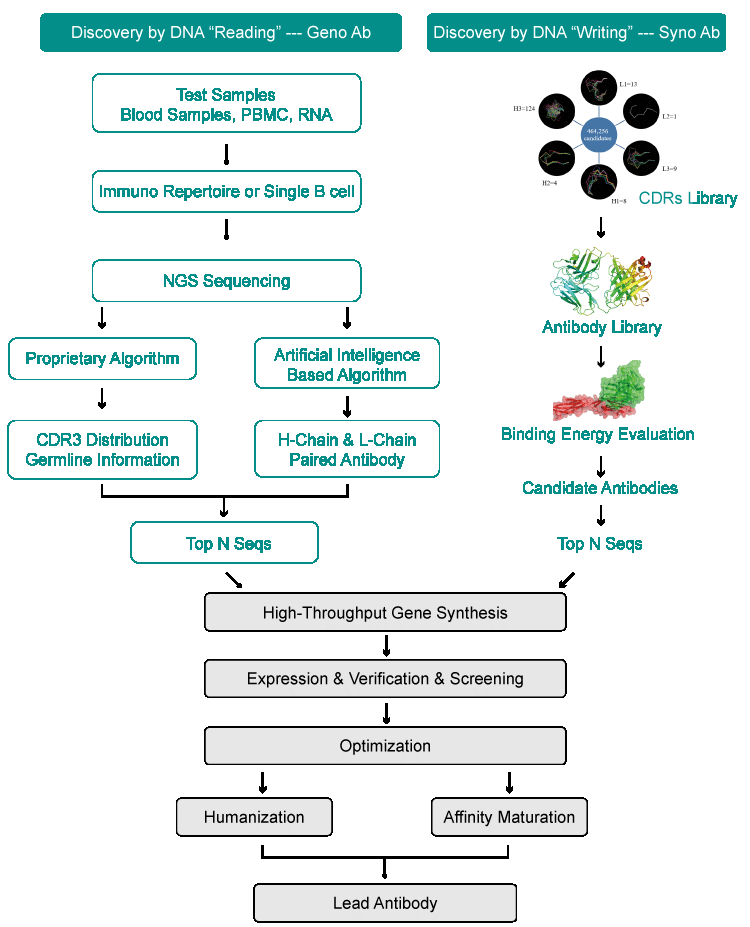
Antibody Services | Synbio Technologies
Synbio Technologies is revolutionizing the development of antibodies through their innovative platform. With a comprehensive, one-stop service offering including gene sequence integration, de novo design, and humanization capabilities - alongside recombinant expression services for both monoclonal and polyclonal preparations - our antibody technology promises to make creating custom antigen solutions easier than ever before.
Our dependable, cost-effective solutions support academia, biotech companies, and pharmaceutical businesses in their research & development projects for therapeutic antibodies. By leveraging our innovative products and services, we strive to facilitate breakthroughs that lead to successful therapies enhanced by synthetically produced components.
When you’re ready to start your next therapeutic antibody breakthrough, you can trust us to help!
Reference
Ng KW, Boumelha J, Enfield KSS,etc. Antibodies against endogenous retroviruses promote lung cancer immunotherapy. Nature. 2023 Apr; 616(7957):563-573.
Kumar, Anand . "Binding mechanisms of therapeutic antibodies to human CD20." Science 369.6505(2020).
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























