In recent years, synthetic biology has been transforming the field of biomanufacturing at an unprecedented pace. As a cutting-edge interdisciplinary field at the intersection of life sciences and engineering, synthetic biology enables the precise design and optimization of biological systems. Through this approach, microorganisms, plant cells, and animal cells can be engineered to efficiently produce target compounds. Its applications span a wide range of industries, including pharmaceuticals, agriculture, chemicals, energy, and food production.
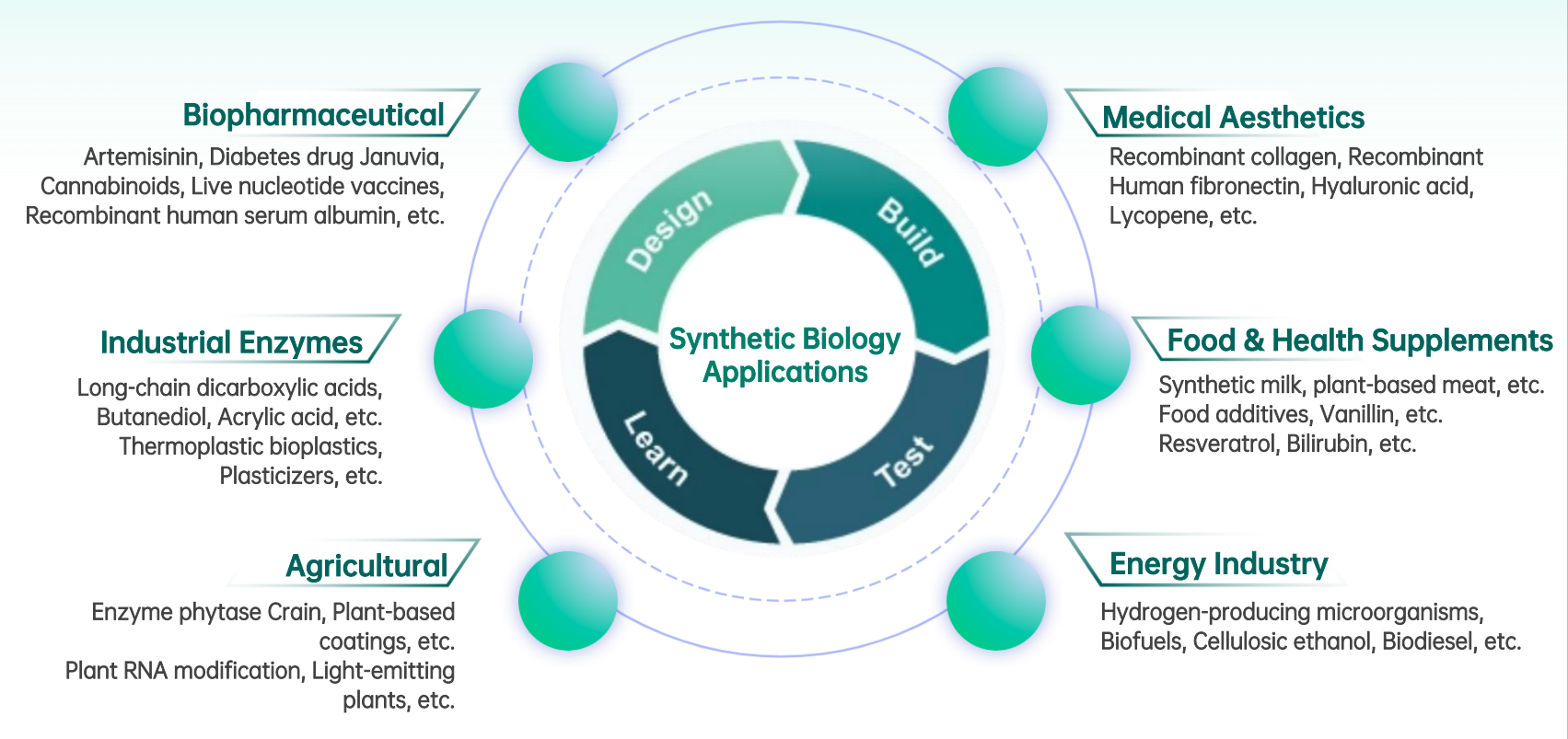
Synthetic Biology Applications
How Does Synthetic Biology Empower Biomanufacturing?
Synthetic biology is revolutionizing biomanufacturing by shifting the paradigm from natural discovery to on-demand design. Through iterative Design–Build–Test–Learn (DBTL) cycles, scientists are continuously engineering and optimizing chassis cells—transforming them into highly efficient biological factories capable of producing a wide array of products, including therapeutic proteins, industrial enzymes, food additives, and biomaterials.
Breakthroughs in DNA sequencing, synthesis, and editing technologies have significantly advanced the "Design" and "Build" phases, accelerating the development of synthetic biology platforms and expanding the pipeline of viable products. Downstream processes such as fermentation and purification have also undergone major innovations, evolving toward greater yield, higher purity, and enhanced product consistency.
However, translating successful laboratory innovations into scalable industrial applications remains a significant challenge. The transition from bench to market often encounters numerous obstacles, including process scalability, economic viability, and quality control. Enhancing production efficiency, reducing manufacturing costs, and improving product quality have become critical goals—and key battlegrounds—for synthetic biology companies worldwide as they strive to achieve commercial breakthroughs.
What’s Holding Back the Industrialization of Biomanufacturing?
Biomanufacturing leverages microbial chassis as biological “chips” to produce a variety of bio-based products through strain engineering, large-scale cell cultivation, and downstream processing. While synthetic biology holds immense promise, translating engineered strains into commercially viable, industrial-scale production systems involves overcoming several critical challenges:
-
Limitations of chassis cell performance: Conventional expression systems such as E. coli and yeast often face issues like low tolerance to metabolic burden, protein degradation, and unstable expression of heterologous proteins. These limitations hinder yield, quality, and process robustness.
-
Complexity of metabolic engineering: Constructing synthetic metabolic pathways involves integrating multiple genetic components. Achieving precise control over metabolic flux is essential to avoid the accumulation of unwanted byproducts and to maximize the efficiency of target compound synthesis.
-
Challenges in scaling up: Strains optimized under laboratory conditions may not perform consistently in industrial environments. Scale-up often leads to issues such as growth arrest, yield reduction, and increased fermentation costs.
-
Regulatory hurdles and market access: In highly regulated sectors such as pharmaceuticals and food, biomanufactured products must undergo rigorous evaluations for safety and efficacy. Lengthy approval processes can delay market entry and increase commercialization risks.
To successfully develop and commercialize synthetic biology products, it is critical to select the right chassis organism and engineer genetic circuits that establish efficient and reliable metabolic pathways—laying the foundation for high-yield, scalable production.
How Chassis Cell Optimization Accelerates Product Development?
Through genetic editing, genetic engineering, and metabolic engineering, scientists can optimize microbial chassis strains to enhance their capabilities in high-yield synthesis, stable expression, and resilience in complex environments—enabling more efficient biomanufacturing.
-
Enhanced Protein Expression: By modifying promoters, optimizing codon usage, and increasing gene copy numbers, target protein expression levels can be significantly boosted to ensure high productivity.
-
Reduced Protein Degradation: Knocking out or suppressing endogenous protease genes minimizes target protein degradation, improving stability and purity.
-
Optimized Metabolic Pathways: Fine-tuning carbon/nitrogen metabolism and auxiliary pathways redirects cellular energy and precursors toward target protein synthesis, maximizing metabolic efficiency.
-
Improved Strain Robustness: Enhanced tolerance to high-density fermentation conditions and reduced metabolic burden from heterologous protein expression prolong the productive growth phase.
-
Streamlined Fermentation: Optimized culture media and key enzyme activity regulation shorten fermentation cycles, lower production costs, and improve industrial scalability.
Synbio Technologies – Synthetic Biology Product Development
Synbio Technologies leverages cutting-edge synthetic biology enabling technologies, including advanced DNA synthesis, gene editing, and proteinengineering, to develop high-performance Pichia pastoris chassis strains for industrial-scale functional protein production.
Our portfolio includes successfully commercialized recombinant proteins such as:
-
Recombinant Collagen
-
Recombinant Human Fibronectin
-
Recombinant Human Serum Albumin (rHSA)
These innovations demonstrate strong market potential across:
✔ Biopharmaceuticals – Next-gen therapeutics & drug delivery systems
✔ Tissue Engineering – Scaffold materials for regenerative medicine
✔ Cell Culture – Serum-free, defined media components
✔ Functional Foods & Cosmetics – Bioactive ingredients with enhanced efficacy
During the development process, we progressed through several critical stages—including strain construction, gene editing, strain screening, and fermentation process optimization. By employing efficient genome editing tools, we successfully knocked out endogenous protease genes in the Pichia pastoris chassis and achieved high-copy integration of target genes. This significantly increased recombinant protein yields while reducing the risk of target protein degradation.
Among various microbial chassis options, Pichia pastoris stands out as a highly promising expression system in the field of biomanufacturing due to its unique biological characteristics. As a eukaryotic organism, P. pastoris offers post-translational modification and protein-folding mechanisms similar to those of mammalian cells—enabling the production of biologically active recombinant collagen.
Furthermore, the co-expression of prolyl-4-hydroxylase in Pichia pastoris facilitates the hydroxylation of proline residues, yielding collagen with a hydroxylation profile closer to that of native human collagen—thereby enhancing its biological functionality. With ongoing optimization of fermentation conditions, recombinant collagen yield has increased substantially, laying a solid foundation for its industrial-scale production.
Synthetic Biology Product Development — Chassis Cell Optimization
Looking for optimized microbial chassis solutions for synthetic biology? At the forefront of synthetic biology innovation, we bring extensive expertise in DNA synthesis, genome editing, and recombinant protein expression.
We offer end-to-end solutions to support your needs in Pichia pastoris strain engineering and protein production—helping you overcome expression bottlenecks and accelerate time-to-market. Whether you’re aiming for high-yield protein expression or precise genetic modifications, we’re here to streamline your R&D journey.
Looking Ahead
In the future, Synbio Technologies will continue to leverage its core competencies in DNA synthesis, gene editing, and protein engineering to expand its portfolio of synthetic biology products. We are committed to delivering more efficient and sustainable biomanufacturing solutions to our global customers.
Guided by our philosophy of open collaboration, we will partner with more organizations to drive the advancement of biomanufacturing technologies. Together, we aim to make meaningful contributions to human health and improve the quality of life for all.
Reference:
1. CHEN Guo-Qiang,WU Fuqing,ZHENG Shuang,DING Jun,SHENG Junting.Current status and applications of microbial chassis strains for Chinese biomanufacturing industry[J].Bulletin of Chinese Academy of Sciences,2025,40(1):2-13.
2. Xiaolei Guo, Yuan Ma, Hang Wang, Hongping Yin, Xinli Shi, Yiqin Chen, Guobiao Gao, Lei Sun, Jiadao Wang*, Yunbing Wang*, Daidi Fan*, Status and developmental trends in recombinant collagen preparation technology, Regenerative Biomaterials, Volume 11, 2024, rbad106, https://doi.org/10.1093/rb/rbad106.
3. Yan J, Yin S, Chen Y, et al. Expression, optimization and biological activity analysis of recombinant type III collagen in Komagataella phaffii[J]. International Journal of Biological Macromolecules, 2024: 138243.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























