Recombinant protein drugs can be used to provide the body with functional proteins that are often lacking due to congenital genetic defects or disease. These innovative medicines use recombinant technology, in which gene fragments incorporated into vectors induce host cells to express specific target proteins – giving rise a new generation of treatments more effective than ever before.
The evolution of genetic recombinant technology marked the beginning of a golden age in the recombinant protein drug industry, with many successful new drugs emerging as research and development shifted to monoclonal antibody and polyclonal antibody medications.
Application of Recombinant Protein Drugs
As biopharmaceutical technologies evolve and advance, recombinant protein drugs are gaining an increasingly prominent role in the treatment of a variety of diseases. These proteins boast increased activity and specificity compared to traditional small molecule drugs, as well as lower toxicity profiles that reduce potential side effects for those undergoing treatment. As such, recombinant proteins have become indispensable components in modern-day pharmaceuticals – ranging from peptide hormones to cytokines, plasma protein factors to enzymes.
| Segmentation | Main Varieties | Treatment Areas |
|---|---|---|
| Recombinant Human Insulin, Insulin Analogues | Diabetes | |
| Peptide Hormones | Recombinant Human Growth Hormone | Childhood dwarfism |
| Recombinant Human Follicle-Maturation Hormone | Promotes female Ovulation |Reproductive Treatment | |
| Cytokines | Recombinant Human Interferon α, β, γ | Hepatitis B, Hepatitis C, and Multiple Sclerosis |
| Recombinant Human Granulocyte Colony Stimulating Factor | Symptoms of various types of cytopenias caused by tumor radiation and chemotherapy, improving patients’ autoimmunity | |
| Recombinant Human Granulocyte Macrophage-Stimulating Factor | Symptoms of various types of cytopenias caused by tumor radiation and chemotherapy, improving patients’ autoimmunity | |
| Recombinant Human Erythropoietin | Symptoms of various types of cytopenias caused by tumor radiation and chemotherapy, improving patients’ autoimmunity | |
| Recombinant Human Thrombopoietin | Symptoms of various types of cytopenias caused by tumor radiation and chemotherapy, improving patients’ autoimmunity | |
| Recombinant Human Interleukin 2, 11 | Symptoms of various types of cytopenias caused by tumor radiation and chemotherapy, improving patients’ autoimmunity | |
| Recombinant Human Epidermal Growth Factor | Traumatic Wound Healing Recovery | |
| Recombinant Human Fibroblast Growth Factor | Traumatic Wound Healing Recovery | |
|
Plasma Protein Factor |
Recombinant Human Coagulation Factor | Hemophilia |
| Recombinant Human Antithrombin | Hemostasis | |
| Recombinant Human Tissue-type Plasminogen Activator | Myocardial Infarction | |
| Recombinant Human Serum Albumin | Plasma Supplementation | |
| Recombinant Human C-reactive Protein | Sepsis | |
| Recombinant Enzymes | Recombinant Human Urokinase Pro | Acute Myocardial Infarction |
| Recombinant Human α-glucosidase Preparation | Pompe Disease | |
| Other | Recombinant Human Bone Formation Protein 2 | Promotes Bone Healing |
| Recombinant Hirudin | Thrombophilia | |
| Fusion Proteins | Oncology and Autoimmune Diseases | |
The Future of Recombinant Protein Drugs
The COVID-19 pandemic has had a large influence on the rapid growth of recombinant protein drugs in recent years. However, many obstacles remain concerning their successful production and development due to them being much more complex compared to small molecule drugs. To achieve mass production of these proteins requires various biosynthetic systems such as those found within bioengineered E. coli, yeast cells, or mammalian cells – all costly endeavors reflective of significant technical and financial barriers that must be overcome for success in this domain.
The rapid advancement of molecular and cellular technologies, in tandem with the introduction of gene editing techniques such as CRISPR-Cas9, has paved a path for significant achievements to be made in vector engineering. As a result, recombinant protein drugs have seen both improved yield quality and hastened processing time – developments that further emphasize their importance within biopharmaceutical production. These advancements have been accompanied by an increasing need for advanced purification processes; from single-column chromatography up to modern online multidimensional chromatographic systems coupled with MCSGP technology – all aimed at improving purity levels and efficiency when manufacturing these vital medicines.
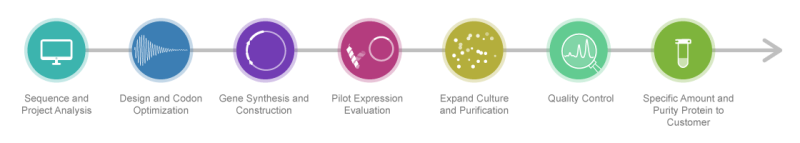
Protein Expression Services at Synbio Technologies
Synbio Technologies is an innovator in protein expression purification, providing scientists worldwide with custom-made milligram to kilogram scale high purity proteins. Our proprietary NG Codon Optimization technology and decade of experience empowers biopharmaceutical R&D for enhanced research outcomes.
Reference
-
Lagassé H A D, Alexaki A, Simhadri V L, et al. Recent advances in (therapeutic protein) drug development[J]. F1000Research, 2017, 6.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products