Protein expression and purification are crucial steps in many fields of biological research and biotechnology. But how do we choose the right methods? Understanding the diverse methods available is essential for obtaining high-quality proteins for various applications.
Four major protein expression systems
One of the most commonly used systems is Escherichia coli. It offers several advantages. It is easy to culture, grows rapidly, and has well-established genetic manipulation techniques. However, it also has limitations. Proteins expressed in E. coli may not undergo proper post-translational modifications such as glycosylation, which is crucial for some proteins' function and stability. To express a protein in E. coli, the gene of interest is cloned into an appropriate plasmid vector. The plasmid contains a promoter, which drives the transcription of the gene, and other regulatory elements. After transformation into E. coli cells, the cells are cultured under specific conditions to induce protein expression. For example, the use of IPTG (isopropyl β-D-1-thiogalactopyranoside) can induce the expression of proteins under the control of the lac promoter.
Yeast, such as Saccharomyces cerevisiae and Pichia pastoris, is another popular choice. Yeast can perform some post-translational modifications similar to higher eukaryotes. Pichia pastoris, in particular, is known for its ability to express high levels of recombinant proteins. The process involves cloning the gene into a yeast expression vector and then transforming the yeast cells. The yeast is cultured in a suitable medium, and protein expression can be induced. The secreted proteins can be easily recovered from the culture supernatant.
Mammalian cells like HEK293 (human embryonic kidney 293 cells) and CHO (Chinese hamster ovary cells) are used when proper folding and complex post-translational modifications are required. These systems are more complex and expensive compared to bacterial and yeast systems. The gene of interest is inserted into a mammalian expression vector with a strong promoter, such as the CMV (cytomegalovirus) promoter. Transfection methods like lipid-based transfection or electroporation are used to introduce the vector into the cells. The cells are then cultured in a specialized medium, and the expressed proteins can be harvested from the cell lysate or the culture supernatant.
Insect Cell Expression Systems:
Baculovirus-infected insect cells, such as Sf9 or Sf21 cells, are utilized. The baculovirus is engineered to carry the gene of interest. Insect cells can produce large amounts of recombinant proteins and are capable of some post-translational modifications. The baculovirus is first propagated in insect cells, and then the recombinant baculovirus is used to infect a large culture of insect cells for protein expression.
What are the methods of protein expression purification?
Affinity Chromatography
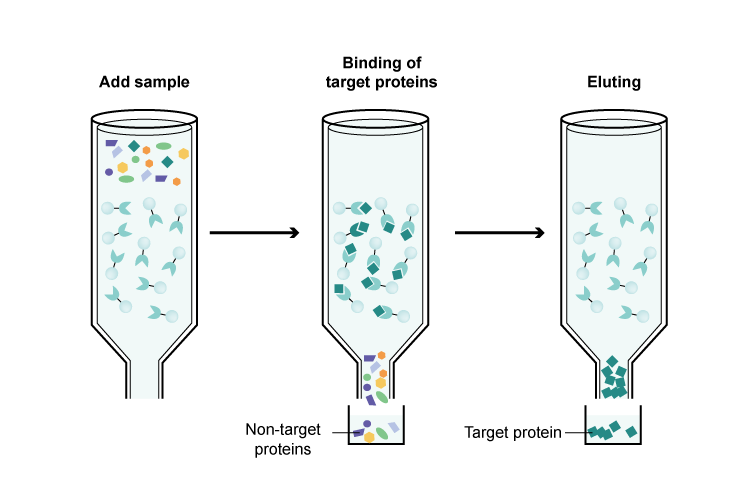
This is a highly specific purification method. It exploits the specific interaction between a protein and a ligand. For example, if a protein has a His-tag, it can be purified using a nickel-nitrilotriacetic acid (Ni-NTA) resin. The protein mixture is passed through the resin column, and the His-tagged protein binds to the nickel ions. After washing away the unbound proteins, the target protein can be eluted using an appropriate buffer, usually one containing imidazole. Another common example is using an antibody - antigen interaction for purification. If an antibody against the target protein is available, it can be immobilized on a resin, and the protein mixture is passed through the column. The target protein binds to the antibody, and it can be eluted under specific conditions.
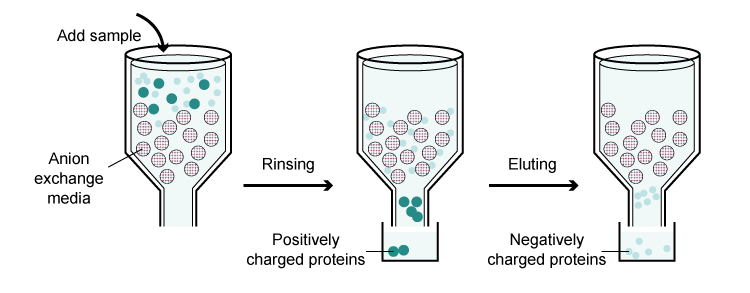
Ion Exchange Chromatography
It separates proteins based on their net charge. There are two types: cation exchange and anion exchange. In cation exchange chromatography, the resin has negatively charged groups, and proteins with a positive net charge at the working pH will bind to the resin. The bound proteins can be eluted by increasing the salt concentration or changing the pH. Anion exchange chromatography works in a similar way but with a positively charged resin and proteins with a negative net charge.
Size Exclusion Chromatography
Also known as gel filtration chromatography, it separates proteins based on their size. The resin contains pores of a specific size range. Larger proteins cannot enter the pores and elute first, while smaller proteins enter the pores and take a longer path through the column, thus eluting later. This method is useful for separating proteins from aggregates or for buffer exchange.
Hydrophobic Interaction Chromatography
It takes advantage of the hydrophobic properties of proteins. The resin has hydrophobic groups. Proteins are first bound to the resin in a high salt buffer, which promotes hydrophobic interactions. Then, the target protein can be eluted by decreasing the salt concentration or adding a more hydrophobic agent.
Synbio Technologies’ Recombinant Protein Expression Systems
Choosing the appropriate protein expression and purification methods depends on various factors such as the nature of the protein, the required post-translational modifications, the desired quantity and purity of the protein, and the available resources.
At Synbio Technologies, all you have to do is supply the protein sequence. We handle everything from Codon optimization and gene synthesis to vector selection, expression system screening, protein purification, andfunctionality validation. With our automated Design-Build-Test-Learn (DBTL) platforms, you’re precisely assured of over 95% success rate.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























