In recent years, the demand for plasmid DNA has surged, driven by advancements in gene therapy, vaccine development, and molecular diagnostics. In this era where biotech innovations are crucial for healthcare, the quality of plasmid DNA is super important. Manufacturing plasmid DNA that meets stringent regulatory and therapeutic standards involves a meticulous process that ensures consistency, purity, and functionality.
Why High-Quality Plasmid DNA is a Big Deal
Plasmid DNA serves as a vital tool in various applications, from genetic engineering to therapeutic development. Its role in delivering genetic material into cells makes it indispensable for applications such as gene therapy, where precise and safe delivery mechanisms are paramount. With the rise of mRNA vaccines, plasmid DNA is even more important as it's used as a template to make mRNA. Thus, ensuring the quality of plasmid DNA is crucial for these biotech products to work well and be safe.
The Plasmid DNA Manufacturing Process
Step 1: Fermentation/Bacterial Culture
The whole process starts with fermentation or bacterial culture. We usually pick Escherichia coli (E. coli) as the host bacterium because of its ability to accommodate and replicate the desired plasmid. The selection of the host bacterium is really important, as it directly influences the yield and quality of the final product.
-
Culture Medium Selection: Culture medium provides the necessary nutrients and conditions for the bacteria to thrive. Common media include LB (Luria-Bertani) broth, which is rich in peptones, yeast extract, and sodium chloride.
-
Cultivation Conditions:The bacterial culture is grown under controlled conditions, including temperature (typically 37°C for E. coli) and pH (usually around 7.0). We keep a close eye on these to make sure the bacteria grow well and the plasmid replicates.
-
Induction Techniques: To maximize plasmid yield, specific techniques like IPTG (isopropyl β-D-1-thiogalactopyranoside) induction may be used. This compound binds to the repressor protein, allowing the expression of genes that promote plasmid replication.
Step 2: Harvesting
Once the bacterial culture reaches the desired growth stage, the next step is to separate the cells containing the plasmids from the culture medium. This process is critical for isolating the plasmid DNA while minimizing contamination and loss.
-
Centrifugation: The most common method for harvesting is centrifugation. The bacterial culture is subjected to high-speed centrifugation, causing the cells to pellet at the bottom of the centrifuge tube. The supernatant, which contains the culture medium and other impurities, is discarded.
-
Alternative Methods:Other physical methods, such as filtration, can also be used for cell separation. These methods are particularly useful for large-scale operations where centrifugation may not be feasible.
Step 3: Initial Purification (Lysis and Clarification)
To refine the extracted solution, a clarification process is employed to remove larger cellular debris. This typically involves centrifugation or filtration, effectively separating solid components from the liquid phase containing the plasmid DNA. Although the clarified solution still contains impurities, it is now better suited for further purification.
Step 4: Fine Purification
Achieving the highest level of purity is essential, particularly for therapeutic applications. Several advanced techniques are utilized in this stage:
-
Column Chromatography:This method capitalizes on the charge differences between molecules. Anion exchange resins selectively bind negatively charged plasmid DNA, enabling separation from other charged particles. This step is crucial for obtaining high purity levels.
-
Gel Filtration/Size Exclusion Chromatography:This technique separates plasmid DNA from smaller contaminants based on molecular size. A gel matrix with specific pore sizes allows larger molecules to elute faster, effectively isolating the plasmid DNA.
-
Affinity Chromatography:Utilizing affinity ligands, this method captures plasmid DNA based on its unique molecular properties. This technique is particularly valuable for achieving high selectivity and purity, making it indispensable in the fine purification process.
Step 5: Formulation and Quality Control
The last step is to make the purified plasmid DNA into a stable solution we can use. Careful adjustments to concentration and buffer composition ensure suitability for its intended application. Quality control is integral at this stage, as adherence to global GMP standards is essential. Comprehensive tests are conducted to verify plasmid concentration, purity, structural integrity, and the absence of microbial contaminants. Ensuring quality not only guarantees product safety but also enhances the plasmid’s performance in therapeutic and diagnostic applications.
Key Takeaways: Why Quality Matters
Plasmid DNA manufacturing is a nuanced, multi-step process that balances scientific rigor with technical precision. Each step, from bacterial culture to quality testing, plays a vital role in delivering plasmid DNA that meets high therapeutic and research standards. As plasmid-based therapies gain traction, high-quality manufacturing processes will continue to influence advancements in healthcare.
For biotech companies and academic researchers alike, understanding these manufacturing intricacies is crucial for achieving successful outcomes in plasmid DNA applications. By sharing this detailed overview, we aim to enhance your understanding of the complex processes involved in plasmid DNA manufacturing, helping you appreciate the science that underpins quality and efficacy in this rapidly advancing field.
Why Choose Synbio Technologies for Plasmid DNA Services?
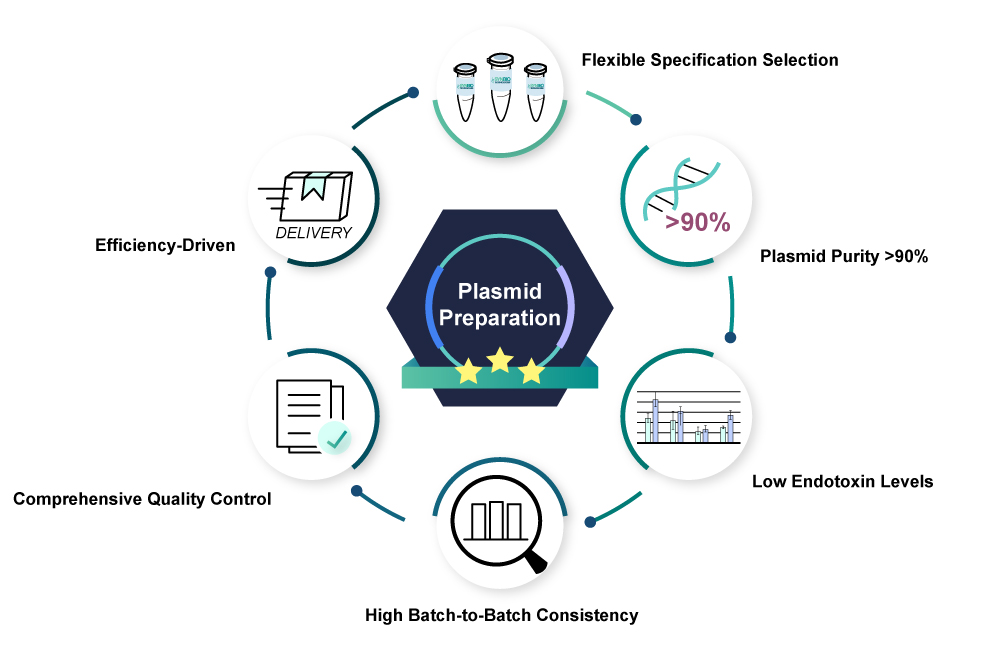
At Synbio Technologies, we specialize in high-quality plasmid DNA preparation tailored to meet your specific research and therapeutic needs. Our synthesized plasmids arehigh quality,free of animal derivatives, and havelow endotoxin content. Whether you’re involved in gene therapy research or vaccine development, our plasmid preparation services are designed to support your innovative projects with reliability and excellence.
References
Abdulrahman, A., & Ghanem, A. (2018). Recent advances in chromatographic purification of plasmid DNA for gene therapy and DNA vaccines: A review. Analytica Chimica Acta, 1025, 41-57.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























