How are plasmids used in gene therapy and genetic medicine?
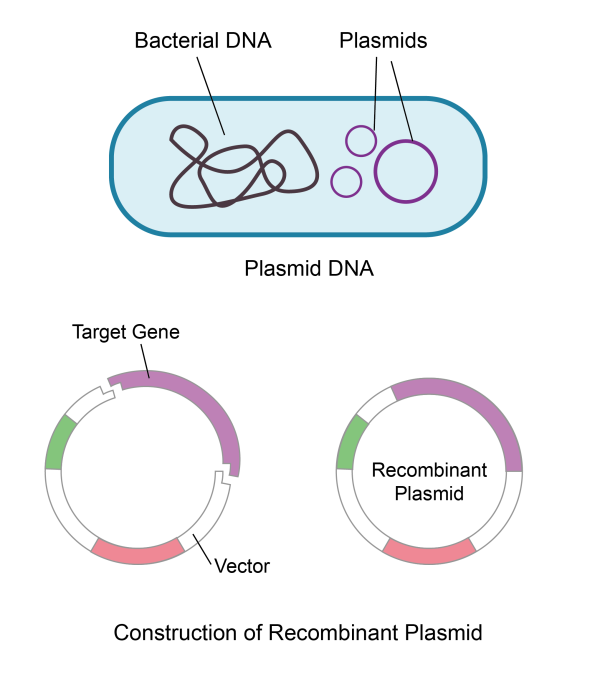
Plasmids, small circular DNA molecules found in bacteria and other microorganisms, are the unsung heroes of molecular biology, genetics, and biotechnology. They provide researchers with an efficient way to study gene function and can carry a wide range of genetic information.
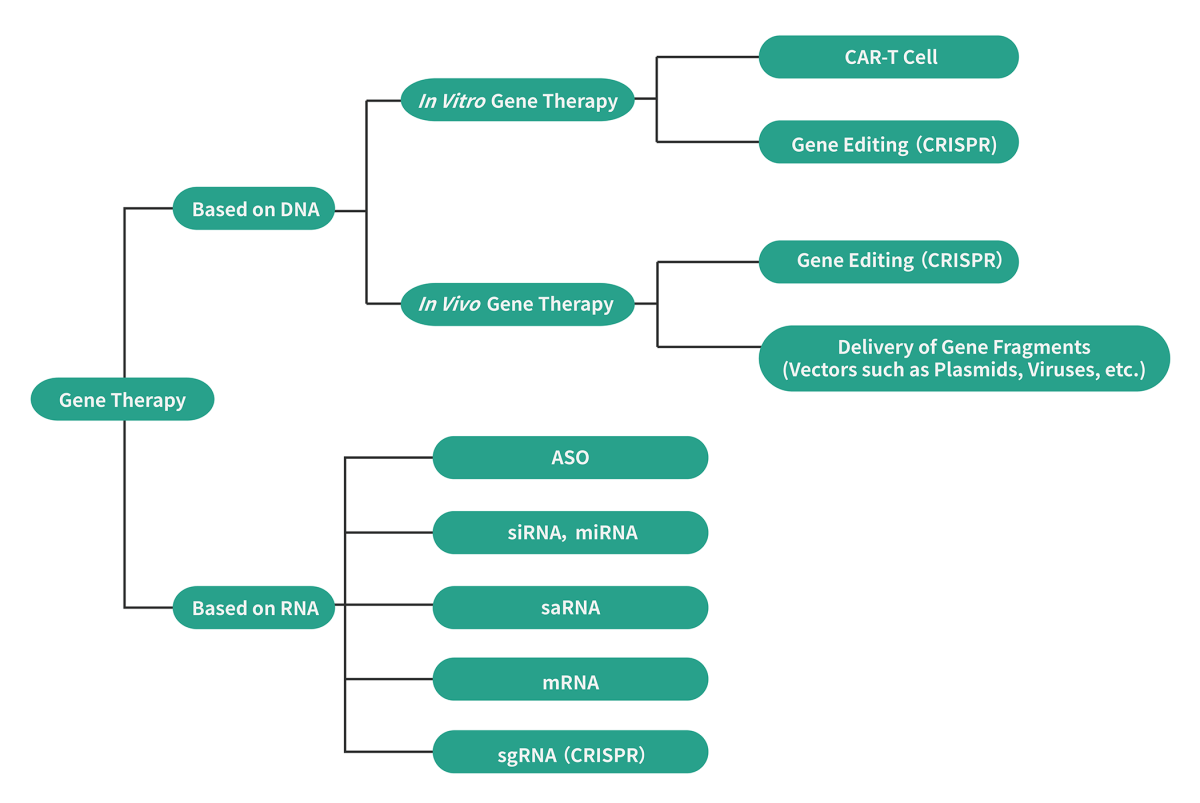
Gene therapy is a cutting-edge medical technique that involves introducing exogenous normal genes into specific cells to address diseases caused by defective or abnormal genes. This innovative form of precision therapy offers the unique advantage of being administered only once, while providing long-lasting and potentially curative effects that traditional medications cannot achieve.
Vectors in Gene Therapy
Delivery of gene therapy drugs is made possible through the use of both viral and non-viral vectors, including plasmids, lentiviruses, adenoviruses, adeno-associated viruses, lysosomal viruses, and lipid nanoparticles. While each vector has its own advantages and disadvantages, recent advancements in this field of research have shown significant promise in treating various diseases, including malignant tumors and chronic degenerative conditions.
Plasmids play a crucial role in gene therapy and DNA vaccine preparation by introducing and expressing targeted genes in specific cells, allowing for the effective treatment and prevention of diseases. Within these applications, plasmids can be utilized in three primary ways:
- Direct injection into the body, such as naked plasmid gene vector.
- In vitro transfection to modify target cells, such as T cells.
- In vitro co-transfection of cells with multiple plasmids to produce viral vectors, such as adeno-associated virus (AAV), lentivirus (LV), and adenovirus.
| Product | Plasmid Requirement | Plasmid Application |
|---|---|---|
| Naked DNA | 20-100μg/Per injection | Direct application of DNA |
| mRNA | 16.67μg/mg mRNA | Using plasmids as templates for production |
| AAV | 0.5mg/L Bioreactor | Requires one personalized plasmid and two generic plasmids |
| LV | 0.5mg/L Bioreactor | Requires one personalized plasmid and three generic plasmids |
| Adenovirus | A few | Amplification by virally infected cells |
According to Cortellis’ data as of September 2022, a total of 1,563 viral and plasmid vectors are used in gene therapy research worldwide, of which 824 AAV vectors are in development and marketed drugs, 203 adenovirus vectors are in development and marketed drugs. However, 106 retroviruses, 54 lentiviruses, 24 herpesviruses, 87 plasmids, and the rest are all unspecified. It is clear that AAV vectors are currently the most frequently used vectors for gene therapy development and utilization, significantly higher than the second most frequently used adenoviral vectors.
As gene therapy gains increasing attention, it faces various opportunities and challenges. These challenges are mainly seen in three areas: the high cost of production, the complexity of genome design and vector optimization, and the limitations posed by animal disease models when exploring new treatments. When it comes to vector optimization, the ideal gene therapy vectors should fulfill specific criteria such as target specificity, stability, low toxicity, large packaging capacity, easy production, and efficient gene transport with long-term expression. To achieve these goals, the production process and quality control of plasmid DNA, a crucial raw material, are of utmost importance.
Why Choose Supercoiled Plasmid DNA?
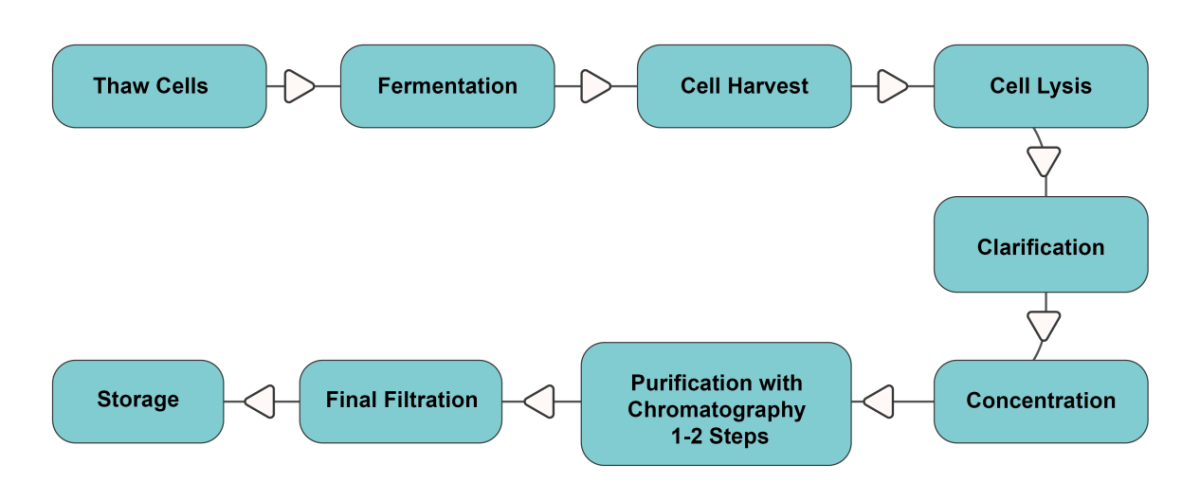
The plasmid DNA production process involves several key steps: constructing the plasmid DNA, thawing cells, fermenting the cells, harvesting and lysing the cells, concentrating the DNA, purifying and filtering it, and finally, storing it. Plasmids are typically fermented and amplified in E. coli, and maximizing the growth density of E. coli can increase the yield of plasmids.
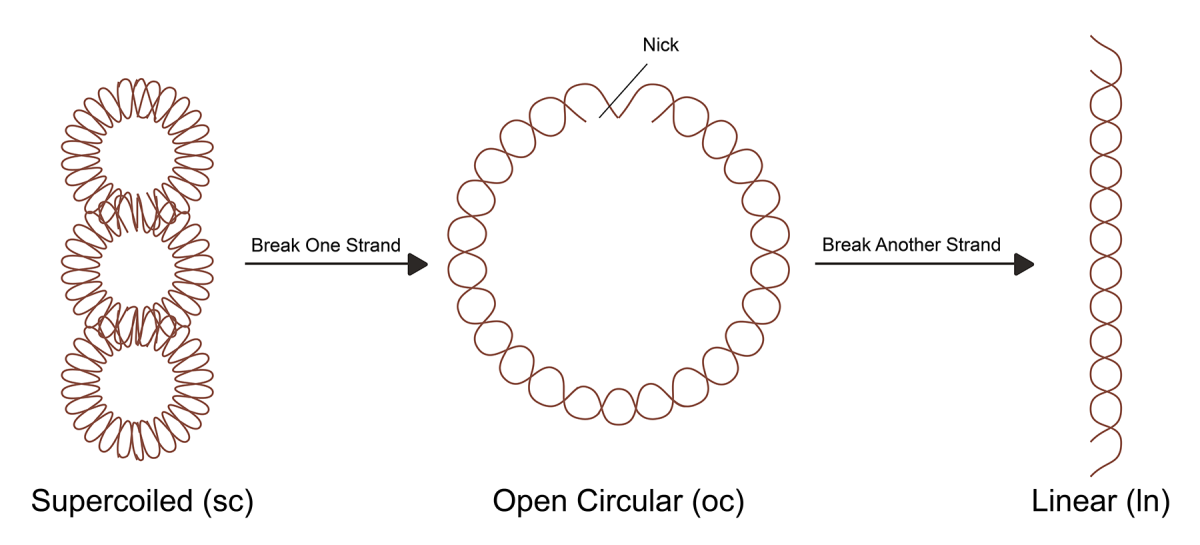
Usually, plasmids from bacteria are circular DNA that exist in a supercoiled (sc) form. During extraction, purification, and storage of plasmid DNA, there are subject to degradation, with single-strand breaks converting the supercoiled structure to an open circular (oc) form and a linear (ln) form. Among these three configurations, supercoiled plasmid DNA is the most stable and has the highest transformation and expression efficiency in cells- which is also a prerequisite to ensure the safety and effectiveness of gene vectors such as naked plasmids, AVV and LV. The US Food and Drug Administration (FDA) has issued guidelines for pharmaceutical plasmid DNA used in DNA vaccines and gene therapy. These FDA guidelines state that the proportion of plasmid DNA that should be supercoiled should be >80%.
Therefore, the biggest challenge in the plasmid production process is to maintain the ratio of high-supercoiled plasmids while maintaining high purity. Plasmid purification plays a vital role in the production process. It eliminates various elements that can impede gene transfection in eukaryotic cells, such as bacterial endotoxins, host bacterial proteins, RNA, proteins, and non-supercoiled plasmids, in order to meet strict quality standards for the final product.
| Standard Plasmid Preparation Package | Research Grade | Transfection Grade |
|---|---|---|
| Optional endotoxin level | <100EU/mg, <30EU/mg, <5EU/mg | <100EU/mg, <30EU/mg, <5EU/mg |
| Quantity | 0.1 mg to Gram Level | 0.1 mg to Gram Level |
| Turnaround time | Starting at 2 Business Days | Starting at 5 Business Days |
| Quality Control Methods | Restriction Enzyme Analysis |
|
| Recommended Applications |
|
|
| Standard Delivery Package | Prepared Plasmid DNA, Certificate of Analysis (COA) and QC Report | Prepared Plasmid DNA, Certificate of Analysis (COA) and QC Report |
Revolutionize Your Research with Plasmid DNA at Synbio Technologies
Maximize your scientific breakthroughs with our cutting-edge plasmid DNA technology and experience the groundbreaking potential of plasmid technology to revolutionize your research. Rest assured, our plasmids are free of RNA and genome contamination. Guaranteeing greater efficiency, our supercoiled plasmids are >90% pure. Additionally, you can choose from three low-level endotoxin options (<100EU/mg, <30EU/mg, <5EU/mg) to meet your specific needs. Trust in our comprehensive, high-quality plasmid DNA preparation services to support your transformative research endeavors. Don’t miss out on this opportunity to unlock the full potential of your research.
Case Study
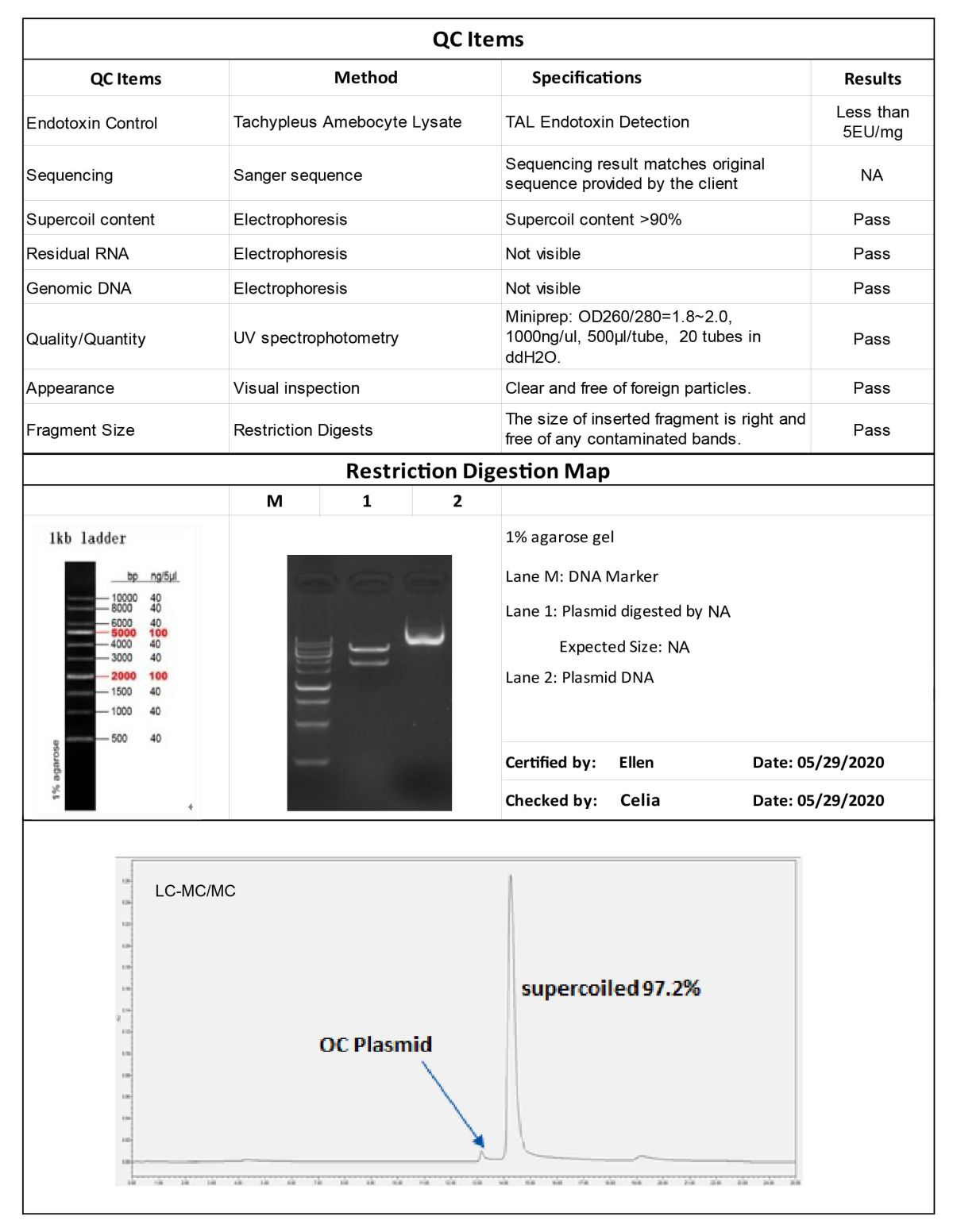
Our strict quality control standards ensure consistent quality, reduce batch-to-batch variability, and meet customer demand for high quality plasmids. From the case data, we show that our plasmid sequencing validation is 100% accurate and has a supercoil content ratio of 97.2%, with endotoxin content control of less than 5EU/mg.
For more information, contact us!
References
- LU XA, HE T, HAN Z, et al. Production of lentiviral vectors in suspension cells using low proportion of supercoiled circular plasmid DNA[J]. Cytotechnology, 2020, 72(6): 897-905.
- HIGH, KATHERINE A., RONCAROLO, MARIA G.. Gene Therapy[J]. The New England journal of medicine, 2019, 381(5): 455-464. DOI: 10.1056/NEJMra1706910.
- DOSS MX, SACHINIDIS A. Current challenges of iPSC-based disease modeling and therapeutic implications[J]. Cells, 2019, 8(5): 403
- Smalla K, Jechalke S, Top EM. Plasmid Detection, Characterization, and Ecology. Microbiol Spectr. 2015 Feb;3(1):PLAS-0038-2014.
- Andersson DI, Jerlström-Hultqvist J, Näsvall J. Evolution of new functions de novo and from preexisting genes. Cold Spring Harb Perspect Biol. 2015 Jun 1;7(6):a017996.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products