Antisense oligonucleotides (ASOs) can pair with target genes to regulate gene expression by specifically blocking the transcription or translation process. The field of ASOs is an emerging area in drug development. Since the concept of ASOs was introduced in 1978, it took over 20 years of development before ASOs were successfully developed into commercially usable drugs. ASO drugs have advantages such as high specificity, high efficiency, and low toxicity, and they are widely used in gene function studies, drug target validation, and cancer therapy.
Marketed drugs for ASOs
|
Drug |
Approval year |
Target Indication |
Target gene |
Mode of action |
Chemistry |
|---|---|---|---|---|---|
|
Formivirsen |
1998 |
CMV retinitis |
CMV |
RNase H1 |
PS-ODN |
|
Mipomersen |
2013 |
HoFH |
ApoB-100 |
RNase H1 |
PS-MOE gapmer |
|
Nusinersen |
2016 |
SMA |
SMN2 intron 7 |
EXON inclusion |
PS-MOE |
|
Eteplirsen |
2016 |
DMD |
Dystrophin exon 51 |
EXON skipping |
PMO |
|
Inotersen |
2018 |
hATTR |
TTR |
RNase H1 |
PS-MOE gapmer |
|
Golodirsen |
2019 |
DMD |
Dystrophin exon 53 |
EXON skipping |
PMO |
|
Volanesorsen |
2019 |
FCS |
ApoC-III |
RNase H1 |
PS-MOE gapmer |
|
Viltolarsen |
2020 |
DMD |
Dystrophin exon 53 |
EXON skipping |
PMO |
|
Casimersen |
2021 |
DMD |
Dystrophin exon 45 |
EXON skipping |
PMO |
Mechanisms of ASOs Drugs on the Market
1. RNase H-mediated degradation: This mechanism is used by PS-ODNs (such as formivirsen) and MOE gapmers (such as mipomersen, inotersen, and volanesorsen). In this mechanism, the RNase H1 enzyme recognizes and cleaves RNA-DNA hybrid structures. To activate RNase H, ASOs must contain 5 to 10 consecutive deoxynucleotides, and PS linkages can also activate RNase H. The gap in the middle of the PS-ODN (8-10 nucleotides) is flanked by 2′-modified nucleotides (such as MOE, LNA, or cEt), which increase stability and affinity, forming a gapmer structure. PS linkages and 2′ modifications reduce RNase H activity, causing these antisense oligonucleotides to act as mere placeholders, thus regulating splicing.
2. Alternative splicing: This mechanism occurs in placeholder oligonucleotides (such as nusinersen and PMO drugs like eteplirsen, golodirsen, viltolarsen, and casimersen), which bind to precursor mRNA and block splice sites, inducing exon inclusion or skipping. This mechanism is used in treating diseases such as spinal muscular atrophy (SMA) and Duchenne muscular dystrophy (DMD).
Clinical Applications of ASOs Drugs
1. Spinal Muscular Atrophy (SMA): SMA is caused by the loss of function of the SMN1 gene. Although the SMN2 gene produces a certain amount of full-length SMN protein, exon 7 is often skipped, resulting in a shortened and unstable protein. Nusinersen, a 2′-MOE-modified PS ASO, promotes the inclusion of exon 7 in SMN2 precursor mRNA, restoring the production of full-length SMN protein.
2. Duchenne Muscular Dystrophy (DMD): DMD is caused by mutations in the DMD gene, disrupting its reading frame and generating premature stop codons, which lead to gene expression interruption. Eteplirsen binds to exon 51 of DMD precursor mRNA and induces its skipping, restoring functional but shorter dystrophin. Similarly, golodirsen and viltolarsen bind to exon 53, while casimersen binds to exon 45, promoting the skipping of these exons and restoring dystrophin expression.
3. Amyotrophic Lateral Sclerosis (ALS): Also known as Lou Gehrig’s disease, ALS is characterized by progressive muscle weakness and wasting, with focal outbreaks spreading to different areas of the body. Patients often die due to respiratory muscle failure. In April 2023, the US FDA accelerated the approval of Tofersen (brand name: Qalsody), an ASO drug for the treatment of SOD1-related ALS. Tofersen induces RNase H-mediated degradation of SOD1 mRNA to reduce the synthesis of SOD1 protein in ALS patients, leading to significant reductions in SOD1 levels in cerebrospinal fluid and the concentration of neurofilament light chain released by damaged neurons in plasma, thereby improving the rate of decline in respiratory function and muscle strength.
4. Hereditary Transthyretin Amyloidosis Polyneuropathy (ATTRv-PN): Transthyretin (TTR) is a plasma carrier protein that transports thyroid hormone and vitamin A to tissues and cells throughout the body. ATTRv-PN is a multisystem disease caused by TTR mutations, primarily characterized by peripheral nerve damage. It involves the instability, hydrolysis, and misfolding of TTR, leading to amyloid fibril deposition outside of cells, damaging tissues and interfering with their normal function. Eplontersen, through its three N-acetylgalactosamine (GalNAc) ligands targeting liver tissues, binds to both wild-type and mutant TTR mRNA, inducing RNase H-mediated degradation to reduce circulating TTR proteins and lower amyloid deposition levels in patients.
Synbio Technologies |ASOs Synthesis Services
Synbio Technologies has established a production workshop that meets the ISO9001 and ISO13485 quality management system requirements. It has standardized production processes and advanced synthesis and purification techniques. All synthesized ASOs undergo strict QC testing, including HPLC purity testing, to ensure the high quality of ASOs products.
Service Highlights
-
ISO 9001 and ISO 13485: Certified quality control ensures reliability
-
Delivery Speed: Guaranteed fast delivery times without compromising quality
-
Customization: Provides various modification types and flexible synthesis specifications
-
Manufacturing Capability: Microgram-to-gram-scale quantity
Service Detail
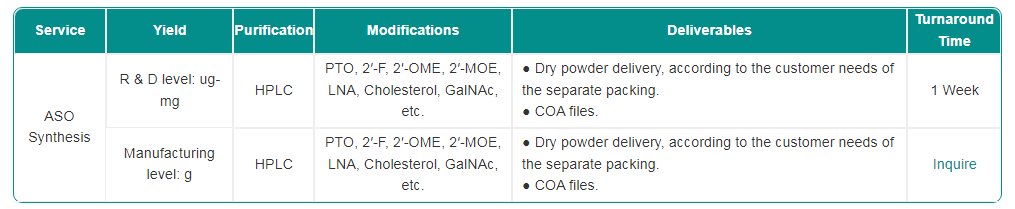
Reference
1. Kim Yeonjoon. Drug Discovery Perspectives of Antisense Oligonucleotides[J]. Biomolecules & Therapeutics,2023,31(3):241-252. doi:10.4062/biomolther.2023.001
2. Echevarría Lucía, Aupy Philippine, Goyenvalle Aurélie. Exon-skipping advances for Duchenne muscular dystrophy[J]. Human Molecular Genetics,2018,27(R2):R163-R172. doi:10.1093/hmg/ddy171
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products
























