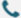Nature’s Molecular Assembly Lines
Biocatalysis is quietly transforming green chemistry and pharmaceutical innovation—evolving from single-enzyme reactions to multi-enzyme cascades. Nature perfected this approach long ago, natural multi-enzyme complexes are not just randomly mixed, but carefully arranged in space and dynamically coordinated. The result? Enabling efficient substrate channeling and precise cascade catalysis.
A classic example is Type II polyketide synthase (PKS). Its core components—ACP (acyl carrier protein), KS (ketosynthase), and CLF (chain length factor)—work in cycles and downstream modifications to build complex, bioactive aromatic polyketides like antibiotics and anticancer precursors.
This principle extends far beyond PKS—many of life’s most essential systems rely on similar multi-enzyme complex drive. The electron transport chain in photosynthesis, the respiratory chain in mitochondria, and the ribosome that assembles proteins are all prime examples. Each is a masterpiece of molecular assembly lines, where efficiency and precision arise not from brute force, but from multi-enzyme complex drive.
Why We Need "Artificially Engineered" Molecular Factories?
Nature’s enzyme assemblies are marvels of evolution—delivering high catalytic efficiency, minimal by-products, and finely tuned control over metabolic flux. These are exactly the features chemists and bioengineers dream of replicating in green chemistry, drug synthesis, biofuels, and environmental remediation. However, natural enzymes have limitations. Natural enzymes are limited by their long evolutionary path and specific physiological functions. That’s why artificial multi-enzyme complexes are gaining attention.
-
Beyond natural limits: Natural complexes struggle with non-natural substrates or entirely new metabolic pathways.
-
Better yield and purity: Nature’s machinery isn’t optimized for Industry-scale production, boost efficiency and robustness requires human intervention.
-
Custom assembly: We need modular, customizable systems tailored for modern multifunctional biocatalytic tasks.
Solving Core Challengesof Biocatalysis
By emulating and surpassing the principles of precise spatial organization found in natural systems, artificial multi-enzyme complexes effectively address the four core challenges pervasive in traditional biocatalysis:low efficiency, insufficient selectivity, poor stability, and high cost.They provide revolutionary tools for developing efficient, precise, stable, and economically viable green biomanufacturing processes.
-
Enhanced Efficiency: Tight assembly reduces diffusion loss of intermediates, significantly accelerating cascade reactions; cooperative multi-enzyme catalysis of multi-step reactions substantially increases reaction rates.
-
Improved Selectivity: Precise spatial control and microenvironment optimization suppress side reactions, enhancing product purity; flexible combination of different enzymes enables multi-step transformations of complex substrates, expanding product diversity.
-
Increased Stability: Modifications or immobilization enhance enzyme resistance to adverse factors like temperature, pH, and solvents; rational design and assembly strategies (such as cross-linking, scaffolding) improve the overall stability and reusability of the complex.
-
Reduced Costs: Optimizing enzyme production lowers unit costs; optimizing reaction processes improves overall economic viability.
Core Challenges and Breakthroughs of Building Artificial Multi-Enzyme Complexes
Constructing efficient multi-enzyme complexes is highly challenging. While direct fusion of enzymes into a single polypeptide may seem like the simplest strategy, this often leads to unexpected problems: misfolded proteins, inclusion body formation, low expression levels, and loss of enzymatic activity. These issues arise largely due to empirical, trial-and-error design—leaving little room for predictability.
To address this, researchers must overcome two fundamental challenges:
Challenge 1: Precise Space Structure-effect Design
The spatial conformation of fusion proteins is inherently uncontrollable. Changes in enzyme orientation or occlusion of active sites can severely impair function. Consequently, computer-aided rational design of fusion sites, linkers, and spatial orientation is crucial. Yet currently, most artificial enzyme assemblies still rely heavily on experimental trial-and-error, due to the lack of computational tools for reliable structural prediction and optimization.
☆ Breakthrough Tool: The iMARS Platform[1]
In January 2025, Prof. Jun Ni’s team at Shanghai Jiao Tong University published a landmark Cell paper titled “Rational Multienzyme Architecture Design with iMARS.”[1]
This research deciphered the "code" behind multi-enzyme systems spatial proximity and efficiency, enabling the rational design of artificial multi-enzyme complexes from sequence to function. The team developed iMARS, a rational design tool with an integrated database of thousands of linkers, allowing rapid prediction of the relative activity of fused enzyme complexes and has demonstrated significant efficacy in multiple application scenarios.
Challenge 2: Efficient Soluble Expression in Host Cells
Artificial multi-enzyme complexes are usually formed by the fusion of multiple enzymes, and the molecular weight is huge. Host cells (such asE.coli and yeast) pose serious challenges to the transcription and translation efficiency of their genes, correct folding and solubility of their expressed products. Unoptimized gene sequences often have extremely low expression levels or form inactive inclusion bodies.
☆ AI Codon Optimization Technology Platform
To tackle poor expression of large molecular weight fusion proteins in host cells, Synbio Technologies launched the NG Codon 2.0 AI-powered codon optimization platform, tailored for artificial multi-enzyme complexes. Its key advantages include:
• AI-powered Codon Optimization
Moving beyond traditional optimization limitations, it leverages advanced artificial intelligence algorithms for global intelligent analysis of target genes. It not only precisely adapts to the codon biases of specific host cells like E.coli and yeast, but also simulates codon usage dynamics and reorders them for optimal translation via deep learning models.
• Enhanced Soluble Expression
Optimized gene sequences promote correct folding of the expressed product and significantly improve soluble expression of the target fusion protein. This reduces inclusion body formation andyields high-activity enzyme complexes.
Synbio Technologies
Ultra-Large Molecular Protein Expression
As an AI-powered leader in synthetic biology, Synbio Technologieshas push the boundaries of ultra-large protein expression with its AI Codon Optimization Platform. Utilizing intelligent codon optimization strategies, the platform successfully enabled high-efficiency expression of a 180 kDa multi-enzyme complex co-expressed with dual chaperones in E. coli. This breakthrough significantly increased the proportion of soluble components by over 35%, overcoming the molecular weight limitations inherent in traditional expression systems.
Looking Ahead
The iMARS Platform[1] solves the challenge of how to design multi-enzyme complexes with optimal spatial configuration.
Meanwhile, Synbio Technologies’ AI codon optimization and gene synthesis platform tackles the bottleneck of how to build and express them effectively. With ongoing advances in artificial intelligence, computational biology, and gene synthesis, artificial multi-enzyme complexes will continue to grow in complexity, functionality, and application scope-ushering in a new era of molecular machine engineering.
Reference
[1] Wang J , Ouyang X , Meng S ,et al. Rational multienzyme architecture design with iMARS[J].Cell, 2025, 188(5):1349-1362.e17. DOI:10.1016/j.cell.2024.12.029.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products