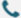Antisense oligonucleotides (ASOs) are a single-stranded oligonucleotide molecule, usually containing 15-25 nucleotides. After ASOs enter cells, under the action of ribonuclease H (RNase H), they bind to their complementary target mRNAs via the principle of complementary base pairing. This inhibits the expression of the target gene. When the expression of the target gene is inhibited, this causes mRNA interference and allows regulation of protein expression.
Antisense oligonucleotide mechanisms can be divided into two types: RNA degradation mechanism and space occupying mechanism. The mechanism by which ASOs promote RNA degradation is through the recruitment of endogenous enzymes, such as RNase H, which recognizes RNA-DNA heteroduplexes and cleaves RNA strands. The binding of the ASO to its target mRNA mimics this DNA-RNA pairing. Therefore, cleavage of the target mRNA by RNase H results in a reduction of the corresponding protein.
Antisense oligonucleotides can be used to inhibit translation or change RNA stability through RNA modification to reduce protein expression. The chemical design of ASOs do not trigger mRNA degradation when paired with target mRNA. For example, ASOs can bind to mRNA and prevent the formation of a 5′-mRNA cap or it can modify the polyadenylation site to prevent mRNA translation or alter RNA stability. Furthermore, ASOs can bind directly to mRNA and sterically block the attachment of 40S and 60S ribosomal subunits along the mRNA transcript during translation. Other ASOs binds to the pre-mRNA intron/exon junctions and directly regulate splicing by masking splicing enhancers and repressor sequences, skipping exons, or forcing the inclusion of other alternate splicing exons.
The development of antisense oligonucleotides for clinical use is challenging because unmodified oligonucleotides are inherently unstable and rapidly degraded by universally expressed endo- or exonucleases. Therefore, some chemical modifications of ASOs can improve the ability of the ASO to recognize target mRNA (target specificity), resistance to nucleases, plasma half-life, and their distribution in tissues.
Phosphate Backbone Modification
The unbridged oxygen atom in the phosphate group of antisense oligonucleotides is replaced by a sulfur atom to form a thiophosphate ester (PS) backbone. The addition of sulfur enhances the nuclease resistance and plasma protein binding of the ASO, which may be due to the increased negative charge density of the sulfur backbone. In addition to better cellular uptake, phosphorothioate backbone modifications support activation of RNase H to break down target mRNAs, a key mechanism of action for many ASOs.
2’ Position of the Sugar Moiety Modification
The most used ASO modification types are 2′-O-methyl (2′-O-Me) and 2′-O-methoxyethyl (2′-MOE), which can significantly enhance nuclease resistance and affinity, potentially providing the highest value in the development of ASOs.
Other Backbone Modifications
ASOs developed by using morpholine instead of ribose rings in oligonucleotides are called morpholino ASOs, which are resistant to nucleases and do not interact with proteins. However, they do not activate RNase H and are therefore primarily used in translation arrest. Recently, several improved modifications and gapmer designs have improved the pharmacokinetic and pharmacodynamic properties of ASOs.
ASOs can not only be used as an effective drug intervention for the treatment of many neurological diseases but can now also be utilized for the treatment of Duchenne muscular dystrophy and spinal muscular atrophy. ASO drugs have been approved by the FDA for marketing and have made outstanding progress in the treatment of Alexander’s disease, hepatitis B, MECP2 repeat syndrome, and other studies.
In the field of neuroscience, the first ASO targeting neuropeptide Y1 (NY1) receptor mRNA was used in the brain in 1993. Specific inhibition of NY1 receptor expression was observed with behavioral changes (anxiety) by repeated injections of this ASO into rat ventricles. Subsequently, it was reported that the ASO targeting rat N-methyl-D-aspartate receptor 1 (NMDA-R1) protein mRNA selectively inhibited protein translation in vivo and prevented neurotoxicity after cerebral ischemia. These results further support the applicability of ASOs to neurological diseases.
In the treatment of facioscapulohumeral muscular dystrophy (FSHD), systematically delivered ASOs for DUX4 transcription was tested in ACTA1-MCM and FLExDUX4 mice expressing DUX4 in the skeletal muscle. Studies have found that DUX4 ASOs were well tolerated in skeletal muscle and inhibited the expression of the DUX4 protein transcript and mouse DUX4 target gene. In addition, DUX4 ASOs can reduce the severity of skeletal muscle pathology and partially prevent inflammation and extracellular matrix genes. Research showed that systemic ASOs targeting DUX4 was a promising therapeutic strategy for FSHD.
Synthesis of Antisense Oligonucleotides by Synbio Technologies
Synbio Technologies has established production workshops that meet ISO 9001 and ISO 13485 quality management certifications, with a standard production process, high-quality synthesis, and accurate purification technologies. Our synthetic antisense oligonucleotides strictly comply with QC testing standards. HPLC purity detection is used to ensure the high quality output of all our antisense oligonucleotide products.
References
[1] Linde F. Bouwman et al. Systemic delivery of a DUX4-targeting antisense oligonucleotide to treat facioscapulohumeral muscular dystrophy. Mol Ther Nucleic Acids. 2021. 26:813-827.
[2] Claudia D. Wursterjavascript:popRef(‘corresp1-1756286418776932’), Albert C. Ludolph. Antisense oligonucleotides in neurological disorders.
Ther Adv Neurol Diso. 2018. 11: 1756286418776932.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries Genome KO Library
Genome KO Library Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard Genome KO Libraries
Standard Genome KO Libraries Standard Genome Editing Plasmid
Standard Genome Editing Plasmid ProXpress
ProXpress Protein Products
Protein Products