Despite the quick evolution of drug innovation, it remains difficult to target certain genomic information with traditional small molecule and antibody drugs. Currently only 1.5% of the human genome is amenable to these types of therapies, while an estimated 80% or more are non-druggable proteins that cannot be targeted by current methods – a substantial hurdle in producing effective treatments for diseases related to recombinant proteins and antibodies. As such, nucleic acid medicines have become increasingly popular as potential solutions due to their ability to address previously unapproachable genetic components underlying disease states.
Nucleic acid drugs boast an impressively wide range of target selection, allowing for their increased potential for success and less vulnerability to drug resistance. Furthermore, these therapies are applicable in a variety of therapeutic areas – making them a promising choice for many conditions.
Nucleic acid drugs are revolutionizing the way we treat diseases, offering treatments that specifically target disease-causing genes or RNA fragments. This novel class of drug has already been used to great effect in genetic and viral illnesses, as well as cancer therapies – with promising results on even more serious conditions. Already, this third generation of medicine is quickly becoming a go-to for doctors worldwide due to its remarkable capacity to address complex medical issues where others may have failed.
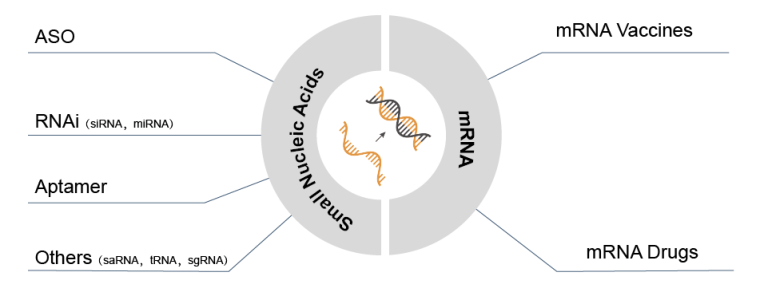
Classification of Nucleic Acid Drugs
Nucleic acid drugs are revolutionizing the way diseases can be treated. Unlike small molecule and antibody medications, they act directly on gene expression to modify disease-causing target genes or RNA fragments, offering a new approach to treating genetic disorders, tumors, and viral infections. This breakthrough is expected to lead nucleic acid drugs to become one of the most common classes of medication in modern medicine.
Development Stages of Nucleic Acid Drugs
In the past 40 years, nucleic acid drug research and development has progressed in leaps and bounds. From its initial exploratory period to a rapid growth stage of advancement achieved through various breakthroughs, this sector is reshaping pharmaceutical standards for generations to come.
Exploration
In 1978, a revolutionary concept was unleashed onto the medical world with the introduction of antisense oligonucleotides (ASOs). Fast forward 20 years, and this breakthrough technology had developed into Fomivirsen, an ASO treatment – now known as Vitravene – that revolutionized healthcare in its approval for sale across the United States.
Slow Development
After Bevasiranib, the pioneering small nucleic acid-based drug, entered clinical trials in 2004 with great expectations for its potential therapeutic use. However, a lack of advanced technology to ensure safe delivery and stability plus mounting safety concerns, caused development of similar drugs to come to an abrupt halt five years later.
Rapid Development
With the continued research and development by companies and scientists around the world, technologies such as the GalNac delivery system and chemical modifications emerged to address the delivery and stability of small nucleic acids.
In a not-so-distant past, the small nucleic acid industry faced stagnation due to limited research and development. However, it was in 2016 when medicine saw an unprecedented rebirth; since then, several revolutionary products have been launched that are capable of addressing rare and chronic diseases – ultimately leveraging the full therapeutic potential of these drugs.

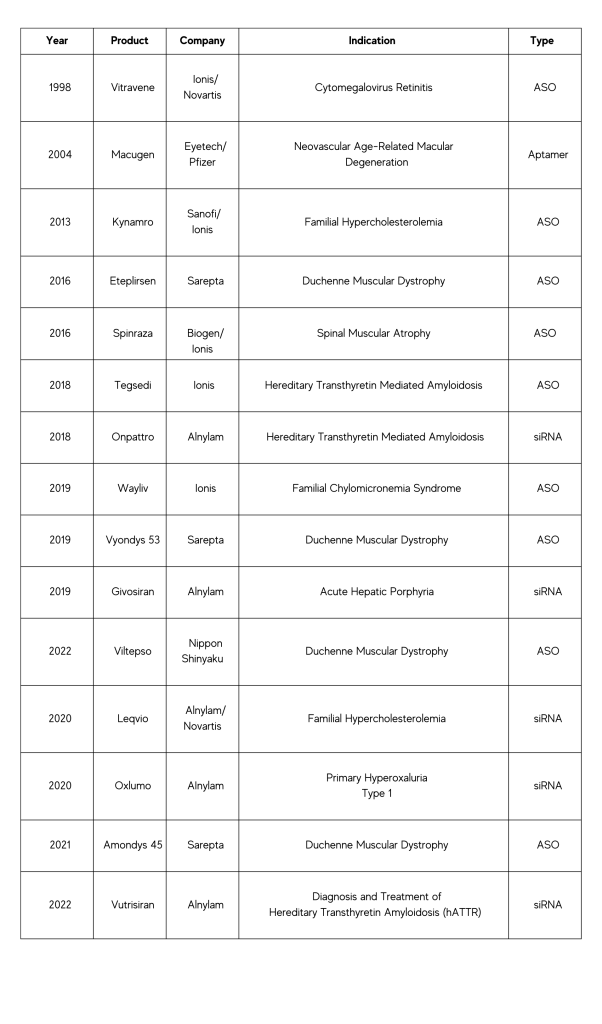
With 15 small nucleic acid drugs having already been approved, the vast potential of this revolutionary class of medicine is becoming more and more evident. Over 100 additional clinical trials are underway with a special focus on ASOs and siRNA, proving to be especially promising avenues for exploration into new medical possibilities.
Nucleic Acid Drugs Approved by the FDA
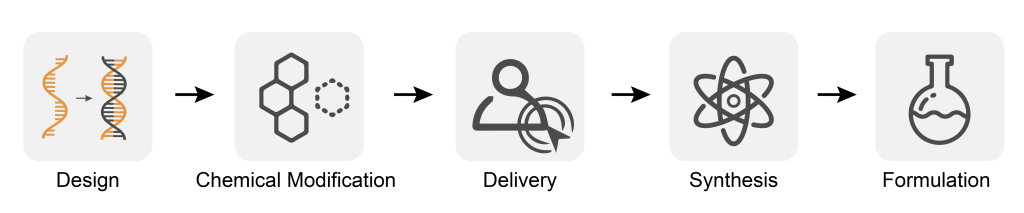
The Challenges of Nucleic Acid Drug Development
In the pursuit of innovative treatments, small nucleic acid drugs are pushing boundaries by taking on complex challenges. The drug development process requires advanced technologies in RNA design, chemical modification, delivery systems, synthesis, and formulation to deliver effective treatments. Despite obstacles encountered along the way, these cutting-edge therapies could lead us towards groundbreaking new solutions for medicine. Small nucleic acid drugs face a formidable challenge in direct delivery to target cells – their large molecular structure, negative charge, and instability can make cell membrane crossing difficult, while enzymes present in serum and tissues are quick to render them ineffective.
Working to address the shortcomings of small nucleic acid drug delivery, R&D teams face significant challenges. Not only must drugs be targeted with precision and safety in mind, but they must also be enhanced for increased activity stability and specificity. Balancing these parameters is critical for successful development of novel therapeutic agents.
Advances in gene sequencing, synthesis, and chemical delivery systems are providing promising pathways to optimize the industrialization of small nucleic acid drugs. Chemical modifications – such as phosphonate acid alterations, ribose changes, and base substitutions – can enhance molecule stability while reducing immunogenicity; key factors for achieving potency within a safe environment. As these developments continue apace, there is real potential for successful clinical translation of this breakthrough technology.
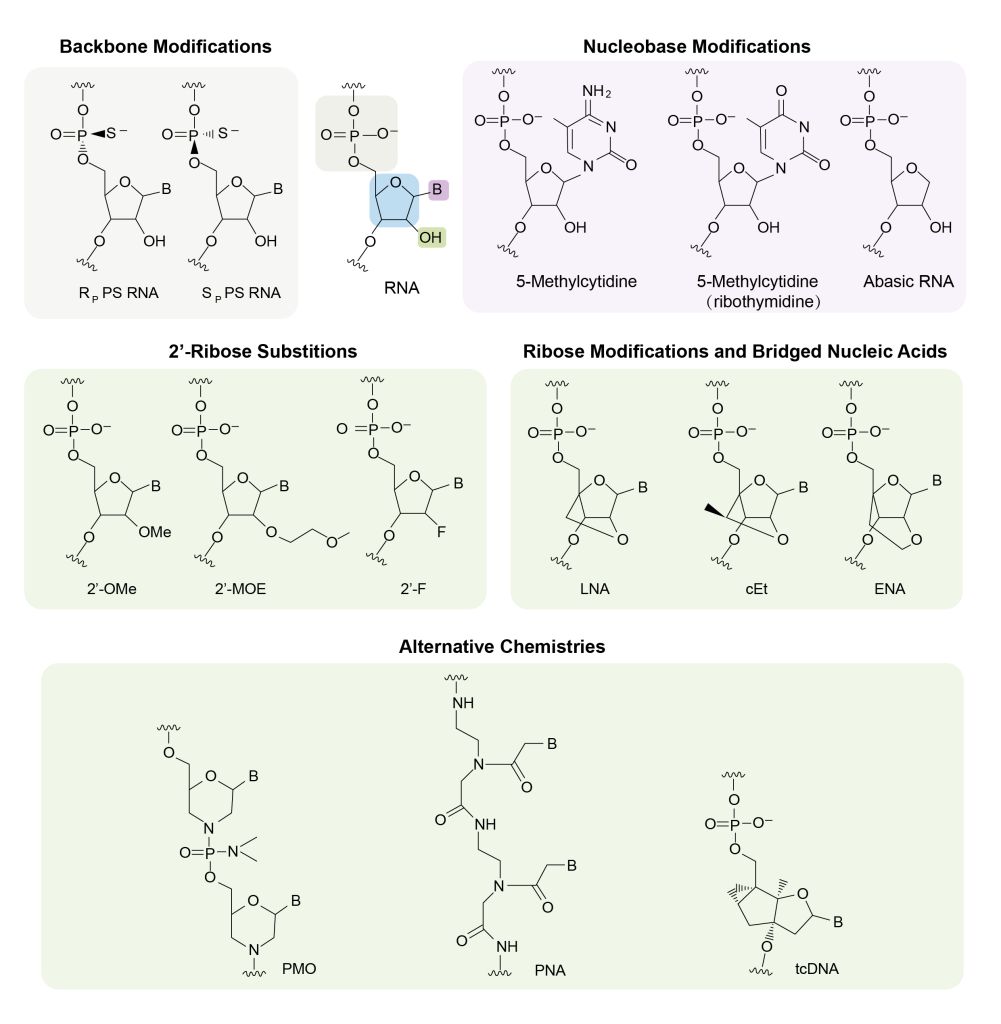
Common Chemical Modifications Used in Oligonucleotide Drugs
Utilizing an efficient delivery system can ensure that nucleic acid drugs reach target cells with efficacy, preventing degradation in the body. Many strategies have been employed to ensure successful and safe transport of these medications into desired tissues or organs including bare RNA modification delivery systems, lipid nanoparticle systems, conjugate optimization delivery systems, and more recently ligand-siRNA conjugates capable of targeting specific receptors. This active targeting reduces risks associated with undesired side effects by limiting drug dosage yet still achieving maximum gene silencing potential at a minimum dose.
GalNAc–siRNA conjugates have emerged as a pioneering nucleic acid drug delivery system, outshining their peers with exceptional efficiency in delivering siRNAs to hepatocytes. This winning combination of GalNAc’s superior targeting properties plus its wide availability has established it as the go-to solution for liver-targeted therapies.
Custom Synthesis Platform for Small Nucleic Acid Drugs
With Synbio Technologies’ drug synthesis platform, scientists have access to an array of nucleic acid drugs and the tools necessary for their research and development. Our proprietary GalNAc delivery system focuses on improving oligonucleotide efficacy and stability, while our analysis services ensure that every step is closely monitored from initial design through chemical modifications all the way up to final product validation.
| Services | Product | Scale | Purification Method | Turnaround Time |
| siRNA | siRNA Synthesis (Unmodified) | µg-g | HPLC / DSL | Contact Us |
| P=S,2-OMe,2’-F,Thiol,Chol,Biotin,FAM,Cy5,Cy3 | ||||
| ASOs | ASOs Synthesis (Unmodified) | µg-g | HPLC | Contact Us |
| PTO, 2’-Fluoro, 2‘-MOE, LAN, Cholesterol | ||||
| Aptamer | Aptamer Synthesis (Unmodified) | µg-g | HPLC | Contact Us |
| Peptide nucleic acid, Chelating agent (NOTA,DOTA,MAG,DTPA), PS , PO, PO/PS , 2’-OMe , 2’-F-RNA, MOE, LNA |
GalNAc–Oligonucleotide Conjugates Platform
Synbio Technologies offers the most effective solution for small nucleic acid drug delivery systems – GalNAc oligonucleotides. By providing optimized conjugate synthesis, our range of products, including Galnac-siRNA, Galnac-ASO, and Galnac-micro RNA, permit efficient and antigenicity-free targeting capabilities for improved therapeutic outcomes.
|
Product |
Scale |
Turnaround Time |
|
Galnac-siRNA |
R & D Level (µg-mg) Manufacturing (g) |
Contact Us |
|
Galnac-ASO |
||
|
Galnac-microRNA |
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries CRISPR Libraries
CRISPR Libraries Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard CRISPR Libraries
Standard CRISPR Libraries Standard CRISPR Plasmid
Standard CRISPR Plasmid ProXpress
ProXpress Protein Products
Protein Products
























