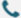Synthetic mRNA provides a template for the synthesis of any given protein, protein fragment, or peptide. With the ease of rapid, large-scale GMP-grade mRNA production, mRNA is ideally poised not only for off-the shelf cancer vaccines but also for personalized neoantigen vaccination. This wiki highlights the advancements in the field of mRNA-based cancer therapeutics, providing insights into key preclinical developments and the evolving clinical landscape.
Structure and Pharmacology of Synthetic mRNA
mRNA was long considered insufficiently stable for pharmaceutical applications, given its susceptibility to rapid degradation by ubiquitous RNases. Through the modification of mRNA non-coding elements (5′ cap structure and its capping efficiency, 5′- and 3′-untranslated regions (UTRs), 3′poly(A) tail) and the coding region, as well as the development of transfection and formulation technologies, the optimization of mRNA has been realized and its wide application has been promoted.
mRNA therapeutics in cancer immunotherapy
In cancer immunotherapy, the most advanced application of mRNA is therapeutic vaccination, which leverages both the capability of mRNA to deliver genetic information and its innate immunostimulatory activity. The latter is particularly important for breaking immune tolerance when cancer-associated self-antigens are targeted.
mRNA is used in anti-cancer vaccination, delivering cancer antigens to APCs for the presentation on MHC class I and II and stimulates innate immune activation by binding to PRRs expressed by APCs, introducing antigen receptors such as CARs and TCRs into lymphocytes, and allows the expression of immunomodulatory proteins including TLRs, chemokine receptors, co-stimulatory ligands, cytokines, chemokines, and different mAb formats in various cell subsets.
With the global threat of the COVID-19 pandemic accelerating rapid-response vaccine development, mRNA-based therapeutics were shown to deliver on their promise: active at a relatively low dose range, can be developed rapidly, and upscaled GMP-compliant manufacturing processes allow rapid availability of large numbers of doses. It is to be expected that lessons learned in the context of COVID-19 vaccine development can be leveraged to further advance the development of mRNA–based cancer immunotherapies.
mRNA in vitro Transcription Service | Synbio Technologies
mRNA is expected to become one of the major pillars of drug development. With an increasing number of concepts entering clinical trials, the first approval of an mRNA therapeutic against cancer is nearing. Synbio Technologies offers optional mRNA modifications such as adding cap structures using mRNA capping enzymes or cap analogs, incorporating modified nucleosides, and adding poly(A) tails to transcribed RNA. For gene editing, you can use the sgRNA synthesized by in vitro transcription to transform the cell along with Cas9 proteins or Cas9 mRNA to perform downstream targeting.
Reference
https://doi.org/10.1038/gt.2010.52.
https://doi.org/10.1186/s12943-021-01348-0.
https://doi.org/10.1016/j.cell.2010.01.020.
 DNA Synthesis
DNA Synthesis Vector Selection
Vector Selection Molecular Biology
Molecular Biology Oligo Synthesis
Oligo Synthesis RNA Synthesis
RNA Synthesis Variant Libraries
Variant Libraries CRISPR Libraries
CRISPR Libraries Oligo Pools
Oligo Pools Virus Packaging
Virus Packaging Gene Editing
Gene Editing Protein Expression
Protein Expression Antibody Services
Antibody Services Peptide Services
Peptide Services DNA Data Storage
DNA Data Storage Standard Oligo
Standard Oligo Standard CRISPR Libraries
Standard CRISPR Libraries Standard CRISPR Plasmid
Standard CRISPR Plasmid ProXpress
ProXpress Protein Products
Protein Products