In December 2019, a new coronavirus (SARS-CoV-2) was discovered due to atypical viral pneumonia cases (COVID-19) in Wuhan. In early January 2020, Synbio Technologies designed and synthesized detection probes for COVID-19 based on our independently developed Syno® qPCR primer/probe design and synthesis platform. In less than two weeks, we completed the synthesis of detection reagent materials, such as amplification primers, probes, and quality controls.
Competitive Advantages
- Speed guarantee: All COVID-19 related orders will be given priority to green channel production, with 24/7 professional online support.
- Quality assurance: ISO 13485 standards of manufacturing, referring to the latest US CDC standards,providing reagents designed with WHO and China CDC standards as well. Low product false positive rate,more cost-effective under the premise of ensuring the quality.
- Production capacity: Experienced synthesis experts, first in class instruments, more than one million probe kit materials supplied per day.
- Product supply: All in one, all 4 sets of primer and probes are included, more scientific and accurate. Virus-related N protein,S protein and other related products are available in stock.
Probe/Primer Synthesis for Virus Detection
According to the official website documents of WHO and China&US CDC, Synbio Technologies has prepared a series of primers and probe materials for COVID-19 detection in batches. We can provide a free trial package and guarantee the supply of a million copies of nucleic acid diagnostic probes daily to speed up disease detection and research.
To help facilitate COVID-19 research and contribute to fight the virus, Synbio Technologies currently has manufactured a detection assay based on CDC Real-Time R T-PCR protocol of detection 2019-novel coronavirus. This assay has successfully detected SARS-CoV-2 in positive control samples N gene and human RNAse P (RNP) gene to assess the specimen quality. It should be considered for research use only (RUO).
SARS-CoV-2 Real Time RT-PCR Detection Assay Specifications
Catalog# SYN202000, this assay kit includes Box #1 and Box #2.
Box #1: Primers and Probes
| Reagent Label | Part # | Description | Quantity /Tube | Reactions /Tube | Price |
|---|---|---|---|---|---|
| SARS-CoV-2_N1 | ST202001 | SARS-CoV-2_N1 Combined Primer/Probe Mix | 37.5 µL/0.56 nmol | 25 | $229/Kit * |
| SARS-CoV-2_N2 | ST202002 | SARS-CoV-2_N2 Combined Primer/Probe Mix | 37.5 µL/0.56 nmol | 25 | |
| SARS-CoV-2_N3 | ST202003 | SARS-CoV-2_N3 Combined Primer/Probe Mix | 37.5 µL/0.56 nmol | 25 | |
| RP | ST202004 | Human RNase P Forward Primer/Probe Mix | 37.5 µL/0.56 nmol | 25 | |
| 2X Reaction Buffer | ST202006 | Buffer, dNTP | 1.2ml | 100 | |
| Enzymes Mix | ST202007 | UDG, Taq DNA Polymerase, Reverse Transcriptase, RNase inhibitor | 200µL | 100 | |
| Nuclease-free Water | ST202008 | Nuclease-free Water | 1.5ml | 100 |
* Each part above can be sold separately, please contact us at service@synbio-tech.com.
Box #2: Positive Control
| Reagent Label | Part # | Descriptions | Quantity | Note | Price |
|---|---|---|---|---|---|
| SARS-CoV-2 Positive Control(Plasmid DNA) | ST202005 | The assays target regions within the SARS-CoV-2 nucleocapsid gene which is present in the SARS-CoV-2_N Positive Control plasmid. The Hs_RPP30 Control contains a portion of the RPP30 gene, a gene present in the human genome. | 125 µL/1 tube | Providing (25) 5 µL test reactions | Price included * |
* Positive Control Plasmid DNA included in Detection Assay Kit, $50 if sold separately.
Features
- Based on CDC protocol, ISO 13485 standards of manufacturing reagents.
- TaqMan™ Real-Time RT-PCR assay primers and FAM probes for higher specificity.
- Targeting three targeted sequence of N gene in GenBank sequences NC_045512.2.
- Positive control included already, no extra preparation required.
Statements
- This assay is for Research Use Only (RUO) and has not been tested on clinical samples.
- This assay is designed based on CDC protocol and public SARS-CoV-2 genome sequences.
- Different sample extraction methods, sample quality, operation parameters, data processing and other un-tested factors might impact this assay’s performance.
- Stability tests and data are not available now due to the emergency. More information will be updated when those are ready.
Certificate of Analysis and Protocol
Primer/Probe Set for WHO
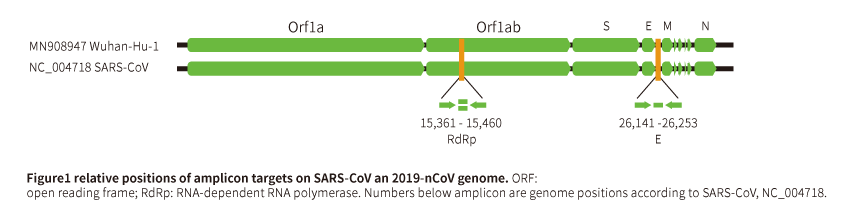
Catalog# SYN20200100, this set includes primers/probes of RdRp, E and N genes.
| Name | Part # | Description | Oligonucleotide Sequence (5’>3’) | Length | Fluorophores/Quencher | Price |
|---|---|---|---|---|---|---|
| RdRP gene | ST20200101 | Primer RdRP_SARSr-F2 | GTGARATGGTCATGTGTGGCGG | 22 | 100rxn/part, $30/set; 500rxn/part, $60/set | |
| ST20200102 | Primer RdRP_SARSr-R1 | CARATGTTAAASACACTATTAGCATA | 26 | |||
| ST20200103 | Probe RdRP_SARSr-P2 | CAGGTGGAACCTCATCAGGAGATGC | 25 | FAM/BHQ1 | ||
| ST20200104 | Probe RdRP_SARSr-P1 | CCAGGTGGWACRTCATCMGGTGATGC | 26 | FAM/BHQ1 | ||
| E gene | ST20200105 | Primer E_Sarbeco_F1 | ACAGGTACGTTAATAGTTAATAGCGT | 26 | ||
| ST20200106 | Primer E_Sarbeco_R2 | ATATTGCAGCAGTACGCACACA | 22 | |||
| ST20200107 | Probe E_Sarbeco_P1 | ACACTAGCCATCCTTACTGCGCTTCG | 26 | FAM/BHQ1 | ||
| N gene | ST20200108 | N_Sarbeco_F1 | CACATTGGCACCCGCAATC | 19 | ||
| ST20200109 | N_Sarbeco_R1 | GAGGAACGAGAAGAGGCTTG | 20 | |||
| ST20200110 | N_Sarbeco_P1 | ACTTCCTCAAGGAACAACATTGCCA | 25 | FAM/BHQ1 |
* Each part above can be sold separately, please contact us at service@synbio-tech.com.
Catalog# SYN20200200, this set includes primers/probes of Target 1(ORF1ab) and Target2(N).
| Name | Part # | Description | Oligonucleotide Sequence (5’>3’) | Length | Fluorophores/Quencher | Price |
|---|---|---|---|---|---|---|
| Target 1 (ORF1ab) | ST20200201 | Forward Primer 1ab | CCCTGTGGGTTTTACACTTAA | 21 | 100rxn/part, $20/set; 500rxn/part, $40/set | |
| ST20200202 | Reverse Primer 1ab | ACGATTGTGCATCAGCTGA | 19 | |||
| ST20200203 | Probe 1ab | CCGTCTGCGGTATGTGGAAAGGTTATGG | 28 | FAM/BHQ1 | ||
| Target 2(N) | ST20200204 | Forward Primer N | GGGGAACTTCTCCTGCTAGAAT | 22 | ||
| ST20200205 | Reverse Primer N | CAGACATTTTGCTCTCAAGCTG | 22 | |||
| ST20200206 | Probe N | TTGCTGCTGCTTGACAGATT | 20 | FAM/BHQ1 |
* Each part above can be sold separately, please contact us at service@synbio-tech.com.
Primer/Probe Set for US CDC
Catalog# SYN20200300, this set includes primers/probes of RP gene and 2019-nCOV N1/2/3 gene.
| Name | Part # | Description | Oligonucleotide Sequence (5’>3’) | Length | Fluorophores/Quencher | Price |
|---|---|---|---|---|---|---|
| RP-F | ST20200301 | RNase P Forward Primer | AGATTTGGACCTGCGAGCG | 19 | 100rxn/part, $40/set; 500rxn/part, $80/set |
|
| RP-R | ST20200302 | RNase P Reverse Primer | GAGCGGCTGTCTCCACAAGT | 20 | ||
| RP-P | ST20200303 | RNase P Probe | TTCTGACCTGAAGGCTCTGCGCG | 23 | FAM/BHQ1 | |
| 2019-nCoV_N1-F | ST20200304 | 2019-nCoV_N1 Forward Primer | GACCCCAAAATCAGCGAAAT | 20 | ||
| 2019-nCoV_N1-R | ST20200305 | 2019-nCoV_N1 Reverse Primer | TCTGGTTACTGCCAGTTGAATCTG | 24 | ||
| 2019-nCoV_N1-P | ST20200306 | 2019-nCoV_N1 Probe | ACCCCGCATTACGTTTGGTGGACC | 24 | FAM/BHQ1 | |
| 2019-nCoV_N2-F | ST20200307 | 2019-nCoV_N2 Forward Primer | TTACAAACATTGGCCGCAAA | 20 | ||
| 2019-nCoV_N2-R | ST20200308 | 2019-nCoV_N2 Reverse Primer | GCGCGACATTCCGAAGAA | 18 | ||
| 2019-nCoV_N2-P | ST20200309 | 2019-nCoV_N2 Probe | ACAATTTGCCCCCAGCGCTTCAG | 23 | FAM/BHQ1 | |
| 2019-nCoV_N3-F | ST20200310 | 2019-nCoV_N3 Forward Primer | GGGAGCCTTGAATACACCAAAA | 22 | ||
| 2019-nCoV_N3-R | ST20200311 | 2019-nCoV_N3 Reverse Primer | TGTAGCACGATTGCAGCATTG | 21 | ||
| 2019-nCoV_N3-P | ST20200312 | 2019-nCoV_N3 Probe | AYCACATTGGCACCCGCAATCCTG | 24 | FAM/BHQ1 |
Synbio Technologies has obtained the following viral RNA through in vitro transcription and prepared positive controls, which is helpful to control the reverse transcription efficiency, achieve the unification of standards, reduce the false negative rate, make the detection results more reliable, and greatly improve the accuracy of the kit detection degree.
| Name | Cat # | Description | Transcription Sequence | Price (50 rxn) |
|---|---|---|---|---|
| RdRP gene | SYN20200401 | RdRP standards | GTGAAATGGTCATGTGTGGCGGTTCACTATATGTTAAACCAGGTGGAACCTCATCAGGA GATGCCACAACTGCTTATGCTAATAGTGTTTTTAACATTTG | $50 |
| E gene | SYN20200402 | E gene standards | ACAGGTACGTTAATAGTTAATAGCGTACTTCTTTTTCTTGCTTTCGTGGTA TTCTTGCTAGTTACACTAGCCATCCTTACTGCGCTTCGATTGTGTGCGTACTGCTGCAATAT | $50 |
| Target 1 (ORF1ab) | SYN20200403 | ORF1ab standards | CCCTGTGGGTTTTACACTTAAAAACACAGTCTGTACCGTCTGCGGTATGTGGAAAGGTTAT GGCTGTAGTTGTGATCAACTCCGCGAACCCATGCTTCAGTCAGCTGATGCACAATCGT | $50 |
| Target 2 (N) | SYN20200404 | N standards | GGGGAACTTCTCCTGCTAGAATGGCTGGCAATGGCGGTGATGCTGCTCTTGCTTTGCTGC TGCTTGACAGATTGAACCAGCTTGAGAGCAAAATGTCTG | $50 |
| Endogenous control | SYN20200405 | RNase P | AGATTTGGACCTGCGAGCGGGTTCTGACCTGAAGGCTCTGCGCGGACTTG TGGAGACAGCCGCTC | $50 |
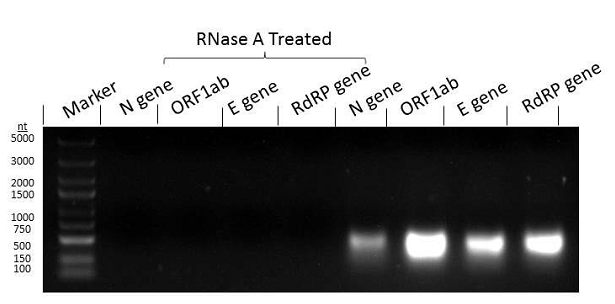
(N-gene part, ORF1ab, E-gen part, RdRP gene part).
*Sequences above were collected from WHO, US CDC and China CDC official website documents. Synbio Technologies’ related products have been added with RNase P, which meets the double detection standard and can greatly reduce the “false negative” phenomenon in the nucleic acid detection process and improve the detection accuracy.

