Are you tired of cloning?
Are you tired of mutagenesis PCR for site mutation, deletion, and insertion?
No worries, we can help. Our Syno® AAV gene delivery service provides one-stop high quality AAV virus. The only thing we need from you is the sequence information of your gene of interest, shRNA or gRNA. We take care of everything from beginning to the end, saving your time, labor, and money. By utilizing yeast homologous recombination technology, together with our NGTM Codon system and Synbio Technologies patent pending codon optimization algorithm, Synbio Technologies is able to provide one-stop services for target gene synthesis, pAAV vector cloning, and AAV virus packaging with your desired scale.
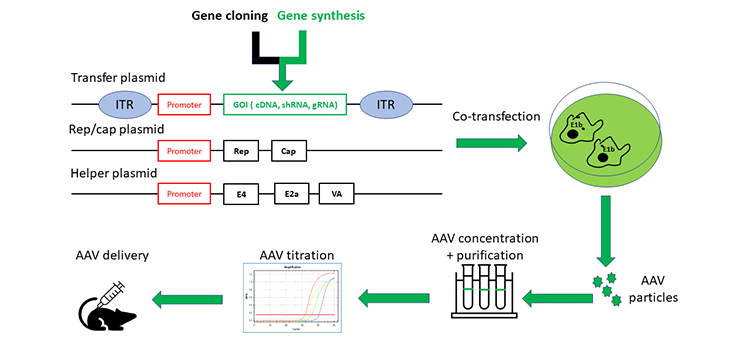
Syno® AAV Production Procedure
Despite its wide diffusion in nature, AAV has never been associated with any human disease to date. It has been proven that AAV is one of the most safe and appealing vectors for in vivo gene therapy. The AAV genome is a 4.8 kb single strain DNA (ssDNA), and consists of two open reading frames, Rep and Cap, flanked by two 145 base inverted terminal repeats (ITRs). Rep codes for the proteins are necessary for viral replication, and cap for the proteins of the viral capsid. The ITR base pairs allow for synthesis of the complementary DNA strand. When constructing an AAV transfer plasmid, the two ITRs are the only AAV sequences preserved in the vectors, while a transcriptional cassette (promoter+ target gene of interest+polyadenylation site) substitutes the rest of the genome, and Rep and Cap are supplied in trans. In addition to Rep and Cap, AAV requires a helper plasmid containing genes (E4, E2a and VA) from adenovirus to mediate AAV replication. The transfer plasmid, Rep/Cap, and the helper plasmid are co-transfected into adenovirus gene E1b+ stable expressed HEK293 cells to produce infectious AAV particles.
Choose Your Right AAV Serotype
AAV2 is the most extensively studied and most widely used AAV serotype. The capsid proteins corresponding to any AAV serotype can recognize the AAV2 ITRs and mediate packaging of the AAV2 genome inside the virions. Thus, it is possible to change the serotype of the vector simply by using, during production, an expression vector for any desired cap gene. Vectors with a capsid corresponding to the AAV1-AAV9, AAV-DJ, and AAV-DJ8 serotypes are commonly generated to exploit the different organ tropism of these viruses. A standard AAV production protocol entails utilization of the AAV2 ITRs in conjunction with the AAV2 rep and serotype specific cap genes.
(√: recommended organ/tissue application)
| AAV Serotype | Muscle | Heart | Liver | Lung | CNS | Retina | Kidney | Pancreas |
|---|---|---|---|---|---|---|---|---|
| AAV1 | √ | √ | √ | √ | √ | |||
| AAV2 | √ | √ | √ | √ | √ | |||
| AAV3 | √ | |||||||
| AAV4 | √ | |||||||
| AAV5 | √ | √ | √ | |||||
| AAV6 | √ | √ | √ | |||||
| AAV7 | √ | √ | ||||||
| AAV8 | √ | √ | √ | √ | √ | √ | ||
| AAV9 | √ | √ | √ | √ | √ | √ | ||
| AAV-DJ | √ | √ | √ | |||||
| AAV-DJ8 | √ | √ | √ | √ |
CNS:Central Nervous System
Competitive Advantages
- High Quality: We guarantee 100% accuracy on all delivered gene products with stringent quality control.
- Highly Flexible Strategies: (1) You prepare your own pAAV vector with gene of interest (GOI), shRNA or gRNA; (2) You provide us GOI, shRNA or gRNA, we subclone into pAAV vector; (3) Synbio Technologies synthesizes gene of interest (GOI), shRNA or gRNA or even whole pAAV plasmid for you.
- One-Stop Service: Our Syno® AAV gene delivery service provides one-stop high quality AAV virus packaging services from gene synthesis to virus packaging to save you time, labor, and money without physically preparing anything yourself.
- Competitive Price: The whole package of one-stop services from gene synthesis, gene construction to virus package with pilot scale (10^12 Genome copies) starts as low as $1,900.
- Technical Support: Professional team extensively-experienced in long gene synthesis and AAV virus production is here for you to support your research.
Service Specifications
| Services | Turnaround Time | Deliverables | Price |
|---|---|---|---|
| AAV Gene Delivery | 2-4 weeks | 2-10 µg transfer plasmid DNA, 200 µl of 10^12 GC (genome copy) | $1,900 |

