Therapeutic antibodies have garnered widespread attention in the biopharmaceutical industry,with promising implications for the treatment of numerous diseases. Advanced humanization techniques for therapeutic antibodies have been identified as a potential game-changer in the life sciences industry, elevating antibody candidates to a new level of prestige, with enhanced qualities such as reduced immunogenicity and superior biochemical and biophysical properties.
The humanization process involves modifying the antibody structure to make it more similar to the ones naturally produced by humans, reducing the potential for adverse immune responses when used as a therapy. With technological advancements rapidly changing the landscape of antibody engineering, humanization is becoming an increasingly attractive approach for the development of safer and more efficient therapeutic antibodies.
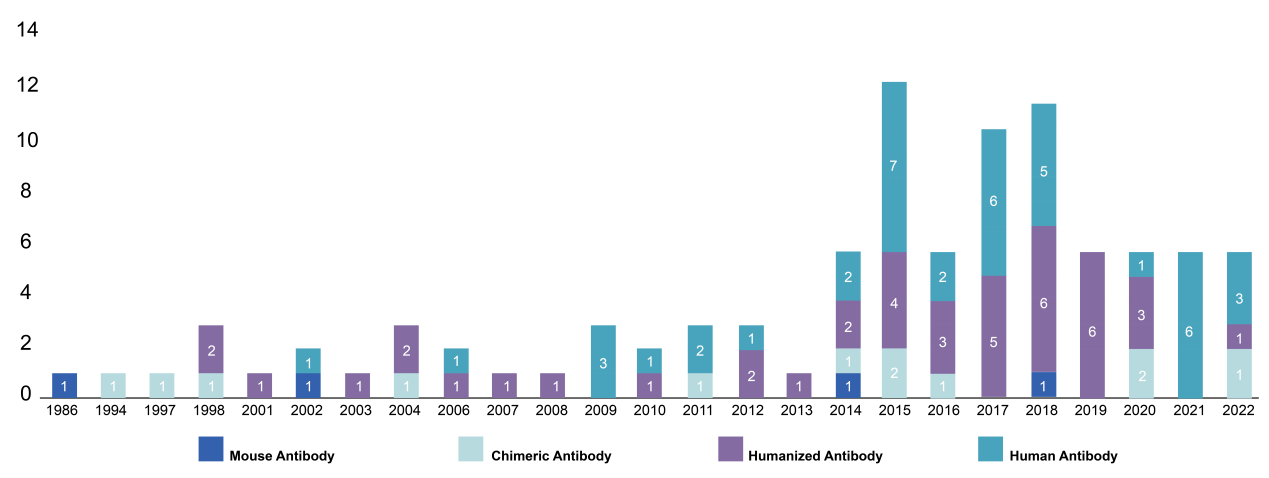
Since the FDA approved the first therapeutic antibody in 1986, Orthoclone OKT3, over 100 recombinant antibodies have received FDA approval. In recent years, the FDA’s approval rate for monoclonal antibody drugs has continued to increase, with a significant focus on humanized and fully humanized antibodies. In fact, all the monoclonal antibodies approved in 2021 are of human origin. This trend suggests that the market will continue to be dominated by humanized or fully humanized antibodies in the future.
Antibody Humanization Approaches
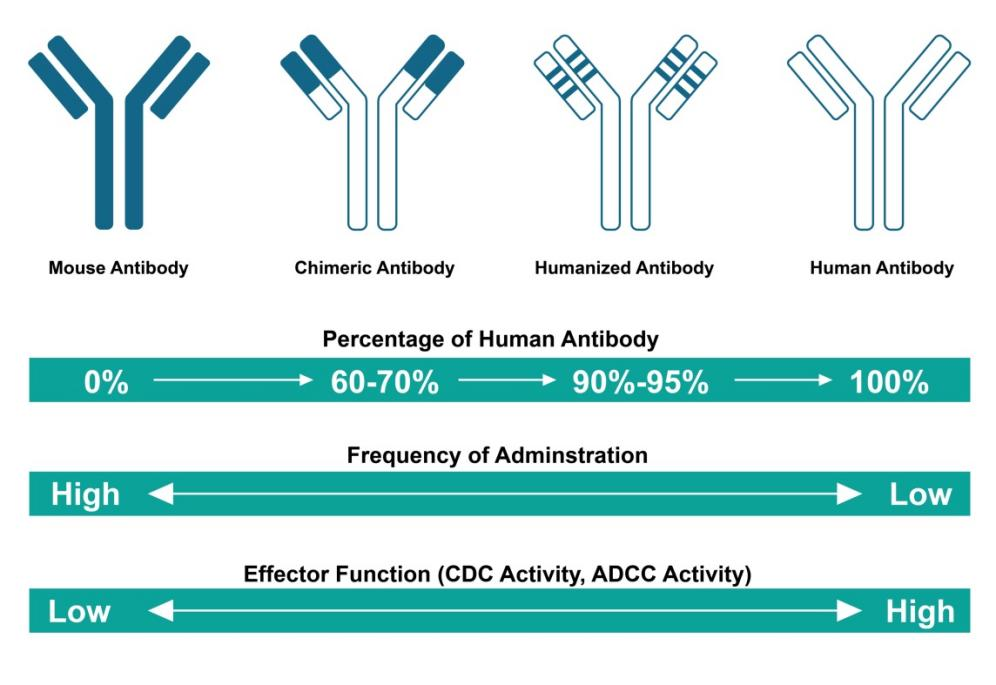
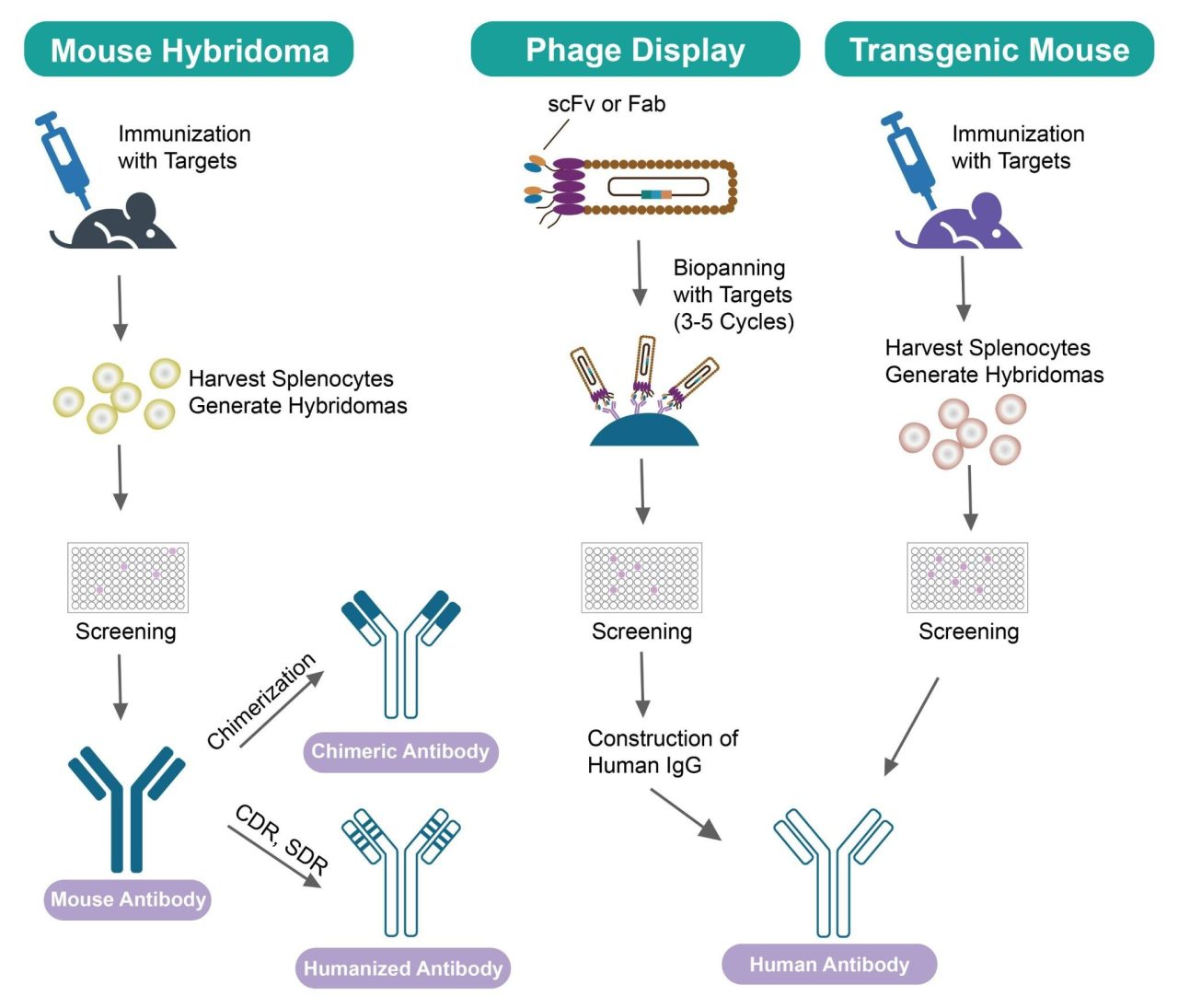
During clinical applications, mouse antibodies prepared by hybridoma technology can trigger human anti-mouse antibody (HAMA) reactions in patients. To address this problem, researchers have used various genetic engineering techniques and computer-aided simulations to optimize the sequence and structure of non-human antibodies to make them more similar to human antibodies. Humanized antibodies can avoid recognition by the immune system, thereby reducing the risk of triggering an immune response and other adverse reactions. The process of antibody humanization consists of three stages: human-mouse chimeric antibodies, humanized antibodies, and human antibodies.
Chimeric Antibody
The generation of chimeric antibodies, which combine mouse variable regions with human constant regions, was the initial approach to lower antigenicity in humans. These antibodies use the antibody variable region from mice and the constant region from humans, with the human amino acid sequence comprising approximately 70% of the entire antibody. Chimeric antibodies preserve the specificity and affinity of the original mouse antibody while significantly reducing the risk of a HAMA response. Some chimeric antibodies are already utilized in clinical settings, but it is important to note that their variable regions are still of mouse origin and may potentially trigger HAMA.
Humanized Antibody
Antibody gene recombination, in which exogenous CDRs are combined with human variable region frameworks, can minimize heterologous non-binding sites while retaining efficient binding of heterologous antibodies to antigens. Antibodies with heterologous and human components expressed through recombinant genes are called humanized antibodies. The degree of humanization of such modified antibodies can reach from 90% to 95%.
- Humanization by CDR-Grafting Method
CDR-grafted antibodies are a modified version of a human-mouse chimeric antibody with further reduction of heterologous components. The variable region of an antibody consists of three complementarity-determining regions (CDRs) and four framework regions (FRs). The CDRs are responsible for recognizing and binding to antigens, determining the antibody’s specificity. While the CDRs vary between different antibodies, the FRs have similar structures. To create humanized antibodies, researchers have incorporated mouse CDRs into the variable region of human immunoglobulin molecules. This allows the humanized antibodies to retain the antigen-binding specificity of mouse monoclonal antibodies while reducing the variability of mouse antibodies. However, this CDR-grafting method can sometimes diminish the antibody’s affinity or specificity for the target antigen. - Humanization by Resurfacing Approach
Surface remodeling antibody technology is used to modify the amino acid residues on the surface of the heterologous antibody FR, resulting in a similar contour to the human antibody CDR and FR frame area. Through this humanization method, antibodies can maintain their selective affinity while minimizing heterogeneity. - Humanization by SDR-Grafting Method
CDR-grafted antibodies may still elicit an anti-idiotypic (anti-Id) response in patients. In order to minimize anti-Id immune responses, researchers have developed a new strategy: specificity-determining region (SDR) grafting. With this method, the antibody can be humanized by grafting only the SDR onto the human frameworks. The SDR is the most critical CDR residue in antibody-ligand interaction and is typically identified either by three-dimensional structures of antigen-antibody complexes or by mutational analysis of antibody binding sites. Grafting the SDR onto the human antibody frameworks can further reduce antibody heterogeneity and minimize the potential immunogenicity of humanized antibodies.
Human Antibody
- Phage-Displayed Antibody Library
Phage-displayed antibody library technology is a powerful tool for antibody humanization, greatly minimizing immunogenetic response in humans. This approach involves replacing mouse VH and VL domains with human variable regions, resulting in monoclonal antibodies with human nature. Additionally, it allows for the direct screening of targeted genes from the library using the antigen, eliminating the need for cell fusion or animal immunization. This technique offers a short experimental period and a simplified process, which is a major breakthrough in the preparation of human antibodies. - Transgenic Mouse
The transgenic mouse technique utilizes genetic engineering to modify mouse models, enabling the mice to produce antibodies encoded by human antibody genes. By doing so, we can overcome the limitations of using animal immunization and humanized antibody technology when developing antibody drugs.
Synbio Technologies | Antibody Humanization Service Platform
Synbio Technologies has developed an integrated antibody humanization and affinity maturation platform based on advanced phage/yeast display technologies as well as computer modeling and simulation techniques. Our team of experts has extensive hands-on experience in humanized antibody design, screening, and production to ensure the success of our clients’ projects. Our integrated platform allows for the humanization of antibodies from a variety of species, including mice, rats, rabbits, camels, birds, and more. We guarantee that the affinity of the final construct is within three times that of the parental antibody.

