Small nucleic acid drugs represent a significant breakthrough in RNA interference (RNAi) technology, offering new horizons in the field of medical therapeutics.
Small nucleic acid drugs encompass a diverse range of molecules, each with unique mechanisms and applications. This includes antisense oligonucleotides (ASOs), which bind to RNA and modulate its function, offering new avenues for treating diseases that were once considered untreatable. Then there’s small interfering RNA (siRNA), a powerful tool in gene silencing that can precisely target and shut down specific genes responsible for disease.
MicroRNA (miRNA) and small activating RNA (saRNA) represent another frontier in RNA-based therapies. While miRNA plays a crucial role in gene expression and regulation, saRNA is involved in upregulating gene expression, offering a novel approach to treating conditions where increased gene activity is beneficial.
Aptamers, often described as chemical antibodies, are another exciting class of small nucleic acid drugs. These short, single-stranded DNA or RNA molecules can bind to specific targets with high affinity, making them ideal for therapeutic and diagnostic applications.
Small nucleic acid drugs, integral to RNA interference (RNAi) technology, encompass a diverse array of molecules including Antisense Oligonucleotides (ASOs), Small Interfering RNA (siRNA), MicroRNA (miRNA), Small Activating RNA (saRNA), and Nucleic Acid Aptamers (Aptamers). These drugs mark a significant departure from traditional pharmacological approaches. Unlike conventional small molecule drugs and antibody drugs, which typically exert their effects by binding to target proteins, small nucleic acid drugs directly target specific mRNA sequences. They achieve this through the principle of base complementarity, intervening in the translation efficiency of mRNA to attain therapeutic effects.
One of the most notable advantages of small nucleic acid drugs is their high degree of target specificity and selectivity. This precision in regulating gene expression opens up new possibilities in gene therapy and gene editing, positioning these drugs as a powerful tool in modern medicine. Their unique mechanism of action and specificity hold immense potential for treating a wide range of diseases, offering a new frontier in medical therapeutics
Small nucleic acid drugs stand apart from traditional small molecule drugs and more recent innovations like monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs). Their unique approach involves regulating target genes to achieve therapeutic effects, offering several distinct advantages:
- Abundant Targets: These drugs act on genes themselves, making it possible to target proteins that are difficult for conventional drugs to reach. This significantly broadens the range of potential therapeutic targets.
- Shorter Development Cycle: By targeting specific pathogenic gene sequences, small nucleic acid drugs allow for more rapid early-stage development. This is achieved through the efficient design and synthesis of corresponding RNA segments.
- Reduced Resistance Likelihood: Targeting at the post-transcriptional level, these drugs intervene directly upstream of protein synthesis. This approach minimizes the chances of developing drug resistance.
- Broader Therapeutic Scope: Small nucleic acid drugs can theoretically target any gene, offering new possibilities for treating diseases currently without effective solutions, including various genetic disorders.
- Long-lasting Effects: Chemically modified versions of these drugs can remain active in the body for extended periods, reducing the need for frequent dosing. This is particularly beneficial in treating chronic diseases.
High Development Success Rate: By identifying disease-causing genes and designing drugs based on gene sequence analysis, small nucleic acid drugs bypass much of the uncertainty in drug development. Their clear mechanisms of action contribute to higher success rates in development.
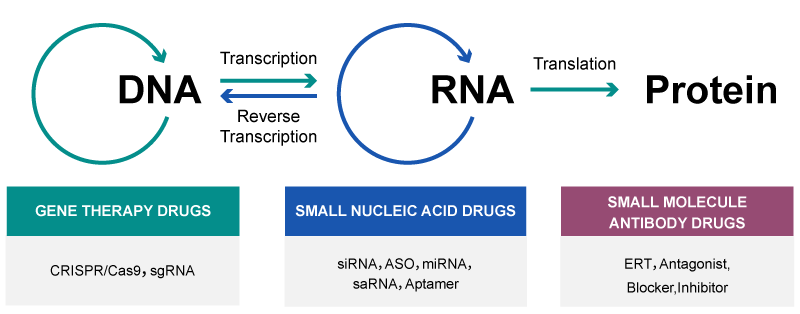
Exploring the Diversity and Mechanisms of Small Nucleic Acid Drugs
Antisense Oligonucleotides (ASOs): These single-stranded oligonucleotides can undergo specific chemical modifications to enhance stability, immunogenicity, and half-life. Their amphipathic nature offers advantages in biological distribution and cellular uptake. Notably, ASOs can be administered as naked nucleic acids, reducing dependence on delivery systems.
Small Interfering RNA (siRNA): A major focus in small nucleic acid drug research, siRNA functions by guiding the silencing complex to precisely cut target mRNA, leading to its degradation or translation inhibition. Due to its hydrophilic nature and weaker binding to plasma proteins, siRNA has a faster clearance rate and thus requires effective delivery systems and chemical modifications for optimal function.
Nucleic Acid Aptamers: These function by using their three-dimensional structures to bind to ligand proteins, thereby regulating protein function. Aptamers, which can be either DNA or RNA, match antibodies in specificity and affinity. They offer benefits like low immunogenicity, reduced production costs, and high-temperature stability.
Emerging Research: The research and development of other small nucleic acid drugs, including miRNA, saRNA, sgRNA, and U1 snRNA, are advancing rapidly, showing promise in various therapeutic applications.
Breakthroughs in Small Nucleic Acid Drug Technology
For exogenous small nucleic acid drugs to be effective, they must overcome several physiological barriers. These include avoiding rapid clearance, resisting degradation by nucleases, enhancing tissue penetration, improving target cell binding, increasing cellular uptake efficiency, and escaping from endocytic vesicles. Historically, these challenges have been significant roadblocks in the development of nucleic acid drugs. However, recent technological advancements have provided effective solutions to some of these issues.
Chemical Modification: Given the inherent instability of nucleic acids and the presence of nucleases in the human body, exogenous nucleic acids are prone to rapid degradation. Furthermore, their immunogenic nature can trigger immune responses. Chemical modifications, including alterations to the ribose, phosphate backbone, bases, and nucleic acid chain termini, can effectively address these challenges.
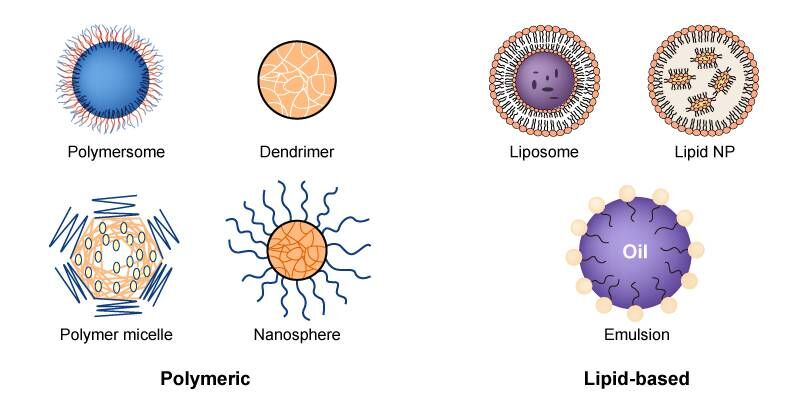
Delivery Systems: Delivery methods for small nucleic acid drugs can be broadly categorized into viral and non-viral vectors. While viral vectors are commonly used in gene therapy, their immunogenicity, potential for tumorigenicity, and limited capacity for drug carriage have restricted their use in nucleic acid drugs. Non-viral vectors, including polymer-based and lipid-based systems, are increasingly popular. These can be further enhanced with specific ligands, such as GalNAc, peptides, and antibodies, for targeted delivery to specific cells.
Advancing Small Nucleic Acid Drug Synthesis with Synbio Technologies
In the rapidly evolving landscape of small nucleic acid drugs, Synbio Technologies stands as a beacon of innovation and excellence. Our proprietary platform for chemical synthesis, combined with our expertise in sequence design, advanced manufacturing, and personalized services, positions us uniquely to offer comprehensive, customized solutions to our clients worldwide. We are proud to contribute significantly to the advancement of this cutting-edge field, setting new benchmarks in medication development. Trust Synbio Technologies for unparalleled precision, speed, and quality in nucleic acid drug synthesis, where every client’s satisfaction is our ultimate goal.