TaqMan probes have revolutionized the field of molecular diagnosis, allowing for accurate and efficient detection of genetic material changes. These probes are short, single-stranded DNA molecules that are designed to bind to specific DNA sequences, enabling researchers to precisely identify and quantify genetic variations in patient samples. TaqMan probes are widely used in a range of applications, from genetic screening and disease diagnosis to forensic analysis and paternity testing. In this blog, we will explore the science behind TaqMan probes, their advantages over other molecular diagnostic techniques, and their diverse applications in modern medicine and beyond.
Core Technologies of Molecular Diagnosis
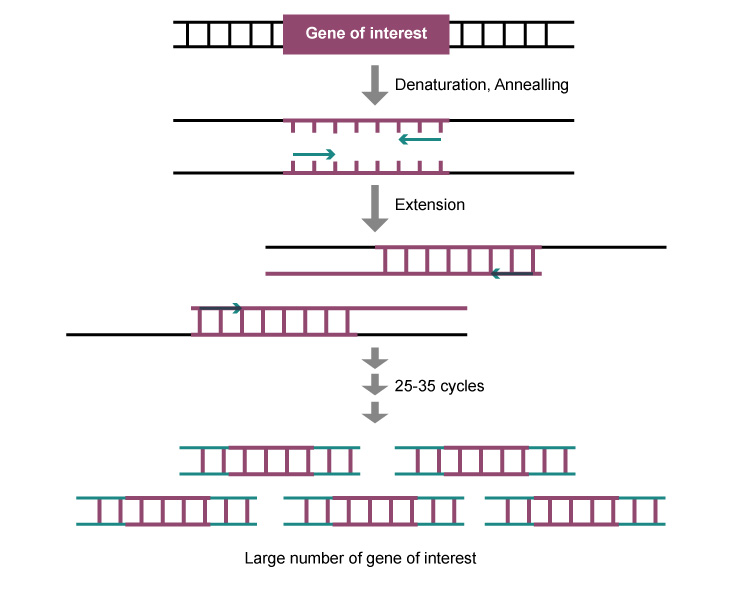
Polymerase Chain Reaction (PCR)
The target DNA fragment is amplified through a cycle of three steps: high-temperature denaturation, medium temperature annealing, and low-temperature extension between the template DNA and the primer.
Advantages:
Low Price, Outstanding Effect on Specific Gene Testing, Easy to Operate
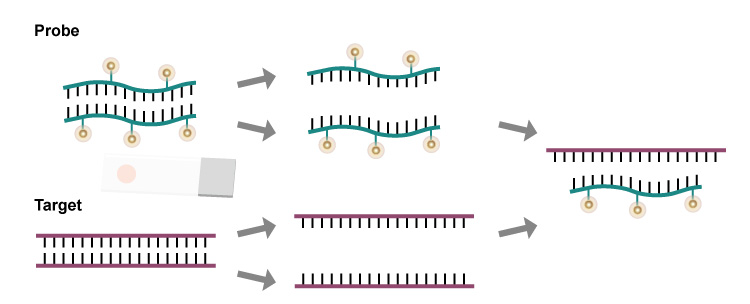
Fluorescence in Situ Hybridization (FISH)
Hybridize nucleic acids in cells or tissue sections using labeled, known sequences of nucleic acids as probes to accurately and quantitatively locate specific nucleic acid sequences.
Advantages:
High Sensitivity, Strong Specificity
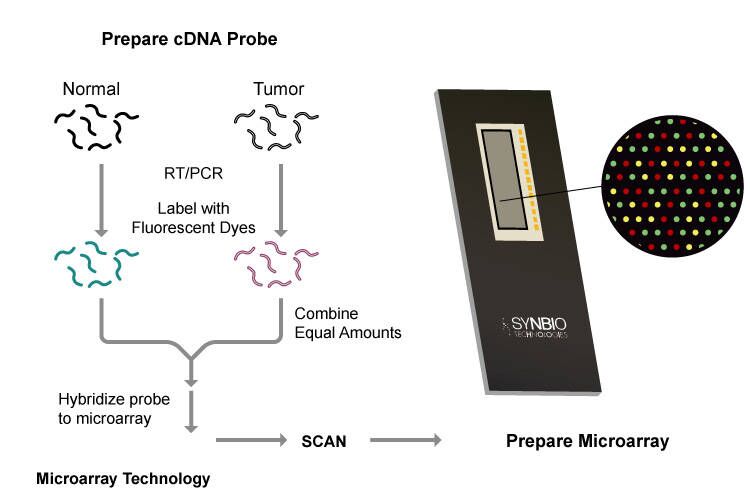
Microarray chip
A probe with a known sequence of target nucleotides is fixed on the surface of a chip, and the sequence is determined by complementary matching.
Advantages:
High Sensitivity, High Accuracy
NGS Sequencing
Anchoring template DNA molecules, base complementary pairing, and reading base sequences by collecting signals
Advantages:
High Throughput, Wide Coverage Area, Long Sequencing Read Length
The world of molecular diagnosis is full of diverse technical paths that offer a wide range of diagnostic capabilities. However, due to the high cost and limited understanding of diseases, the advancement of diagnostic techniques has been slow. Currently, there are four main types of molecular diagnosis: PCR, sequencing, microarray chip, and hybridization. Among these, PCR is the most commonly used technique due to its low cost, high specificity, and ease of use. However, the other three techniques are also showing great potential and are currently in the process of development and expansion. As these technologies continue to evolve, they will offer new possibilities for the diagnosis and treatment of a wide range of diseases.
Real-Time PCR: The Advantages and Types of qPCR Methods
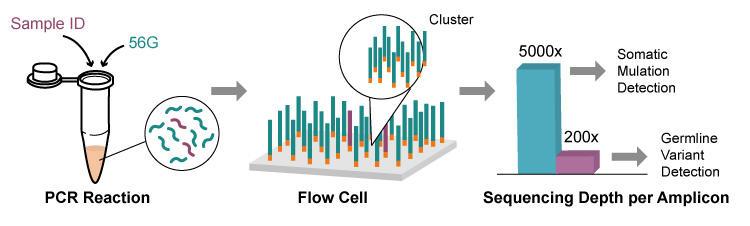
Real-time PCR, also known as qPCR, is a modification of traditional PCR that incorporates fluorescent signals for PCR amplification detection. This technique not only inherits the advantages of traditional PCR, such as high sensitivity and ease of use, but also allows for quantitative detection of samples. There are two main types of qPCR methods: dye and probe methods. The dye method is less expensive but has a higher risk of producing false positives, similar to the interference of ordinary PCR amplification products. The probe method uses probes, such as TaqMan probes, which can overcome the non-specificity issues present in the dye method. TaqMan probes can specifically bind to the target DNA sequence, resulting in a more accurate and reliable detection method.
TaqMan Probes: Working Principle and Applications in PCR Amplification
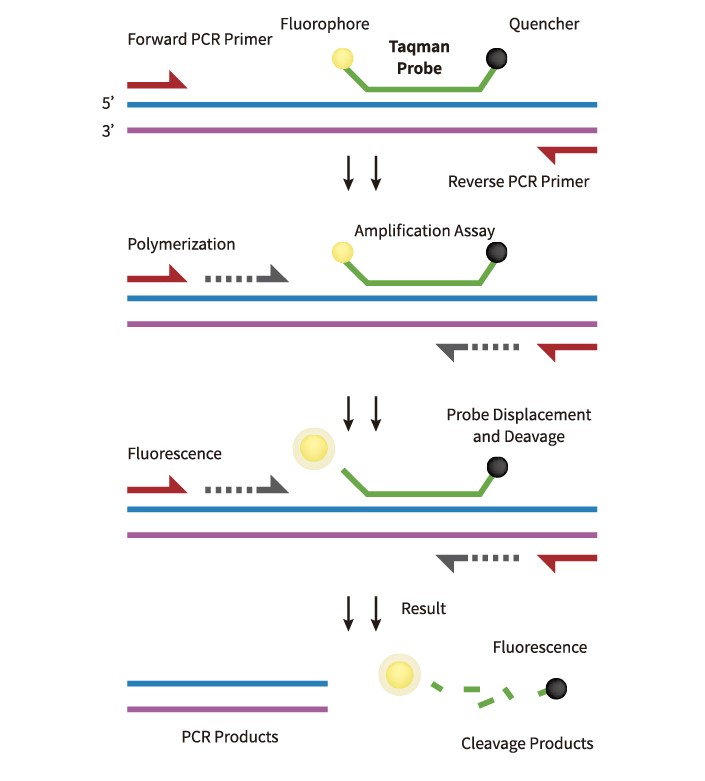
The TaqMan probe is a type of probe commonly used in PCR amplification. It is a dual-labeled probe that contains a fluorescence reporting group at the 5′ end and a fluorescence quenching group at the 3′ end. During the PCR process, the Taq enzyme cleaves the probe that binds to the target sequence, which separates the reporting group from the quenching group. As a result, fluorescence signals are released, and the sample can be quantitatively analyzed by detecting increases in fluorescence intensity during the amplification process. The fluorescence reporting groups that can be used with TaqMan probes include HEX, FAM, ROX, JOE, and VIC, among others. The fluorescence quenching groups mainly include TAMRA and BHQ, which are used to prevent probe extension during PCR amplification. The specific design of the TaqMan probe ensures high specificity and sensitivity for target sequence detection, making it a popular choice in many applications, including gene expression analysis and genetic disease diagnosis.
Advantages of the TaqMan Probe Method in Molecular Diagnostics
The TaqMan probe method is a powerful technique that combines the efficiency of PCR amplification, the specificity of probe technology, and the sensitivity of spectral technology. This method overcomes the limitations of traditional PCR, which can only achieve qualitative detection. The TaqMan probe method allows for quantitative detection, enabling researchers to determine the exact amount of target DNA in a sample. It is a fully-enclosed test tube operation, which reduces the occurrence of false positives and avoids environmental pollution. Compared to traditional PCR electrophoresis, the results are more objective and can be determined as positive, negative, or suspicious, ensuring the accuracy of the results. The TaqMan probe method also reduces the minimum detection limit of the system, making it 1-2 orders of magnitude more sensitive than ordinary PCR. These advantages make the TaqMan probe method an essential tool in molecular diagnostics, allowing for precise and reliable detection of genetic material changes in patients.
Design Principles and Precautions for TaqMan Probes in PCR Amplification
Designing TaqMan probes requires careful consideration of various factors to ensure optimal performance and accurate results. Here are some principles and precautions to keep in mind when designing TaqMan probes:
- Selecting the Appropriate Probe Sequence: The probe sequence should match the specific region of the target sequence as closely as possible to avoid binding with non-target sequences. Choosing a sequence closer to the 3′ end can improve the stability and sensitivity of the probe.
- Choosing the Appropriate Length: The probe length should be between 20-30 nucleotides. Probes that are too long or too short can affect their specificity and stability.
- Determining the Tm Value of the Probe: The Tm value of the TaqMan probe should be approximately 10°C higher than that of the primer, which is typically between 65-70°C. This helps ensure that the stability of the probe-template binding is greater than the stability of the primer-template binding, allowing the probe to bind to the target fragment before the primer during annealing.
- Optimizing Probe and Primer Concentrations: Achieving optimal probe and primer concentrations is crucial to prevent any adverse effects on PCR amplification efficiency and specificity. The ratio between them should be carefully balanced, and the probe concentration should be optimized within the range of 50-250 nM. Similarly, the primer concentration should be optimized within the range of 50-900 nM to ensure the best possible outcome.
- Avoiding Repetitive Nucleotide Sequences: Repetitive nucleotide sequences, especially G (no 4 consecutive G bases), should be avoided for efficiency and reproducibility.
Following these principles and precautions when designing TaqMan probes can help ensure that the probes are specific, sensitive, and produce accurate results.
TaqMan Probes in Medical Diagnosis: Detecting Gene Mutations for Personalized Treatment
TaqMan probes have a wide range of applications in medical diagnosis, particularly in detecting gene mutations related to specific diseases. For example, in breast cancer diagnosis, HER2 gene amplification is a common genetic variation that can be detected using TaqMan probe technology. By detecting HER2 gene amplification, diagnosticians can determine whether patients need HER2 targeted therapy and monitor the variation of HER2 gene amplification, providing a basis for personalized treatment of breast cancer.
In HER2 gene amplification detection, TaqMan probe technology leverages the principle of fluorescence resonance energy transfer (FRET). Two probes, labeled with different fluorescent groups, are designed based on the specific region of the HER2 gene amplification. One probe detects the target sequence, while the other detects the reference sequence. These probes are then reacted with DNA fragments in the sample to be tested, and changes in fluorescence signals are used to determine the presence of the target sequence. If the target sequence exists, the fluorescence signal will change, indicating the amplification of the HER2 gene.
With TaqMan probe technology, diagnosticians can obtain highly-accurate HER2 gene amplification detection results in a short time, enabling more accurate and personalized solutions for breast cancer treatment. TaqMan probes also have applications in detecting other gene mutations related to various diseases, making them an essential tool in molecular diagnostics.
Synbio Technologies: Your Trusted Partner for TaqMan Probe Synthesis
Synbio Technologies is a leading provider of nucleic acid probe synthesis services, including high-quality TaqMan probes. Our expertise and state-of-the-art production processes ensure that our TaqMan probes meet the highest standards of quality, accuracy, and efficiency.
We offer customized design and synthesis services to meet your unique research needs, allowing you to personalize your probes with various fluorescent groups labeled at the 5′ or 3′ ends or at designated locations. With our precision and confidence, you can enhance your research and unlock its full potential.
Partnering with Synbio Technologies for TaqMan probe synthesis means gaining access to cutting-edge technology and expert support every step of the way. Trust us to help you achieve your research goals and revolutionize your experimental accuracy and efficiency.
Service Advantages
-
-
- High Quality: Probe purity can reach over 98%, low background noise
- Strict Quality Control: ISO 9001 & ISO 13485 quality management system certifications
- High Purity: Efficient removal of small molecule fluorescent dye residues, 100% LC-MS quality inspection
- Customization: Imported raw materials, synthesis specifications from nm to μm level
-
The Future of TaqMan Probe Technology in Molecular Diagnostics
TaqMan probe technology has become the core technology of molecular diagnostics, offering remarkable sensitivity and specificity for detecting gene mutations related to various diseases. This powerful technique continues to evolve, with newer and better probe such as dual-quenching probes and TaqMan-MB. With advancements in science and technology, we can expect even more precise and rapid PCR amplification instruments, paving the way for superior primers and probes in the process. Combined with continuously innovative software analysis technology, the TaqMan probe method is set to continue its position as a leading diagnostic tool. Its widespread implementation and further advancements promise to transform the way we diagnose and treat diseases in the years to come, leading to better patient outcomes and improved public health.
Reference
- Qiu L, Chen X, Guo X M, et al. A TaqMan probe based real-time PCR for the detection of Decapod iridescent virus 1[J]. Journal of invertebrate pathology, 2020, 173: 107367.
- Huang X, Chen J, Yao G, et al. A TaqMan-probe-based multiplex real-time RT-qPCR for simultaneous detection of porcine enteric coronaviruses[J]. Applied microbiology and biotechnology, 2019, 103: 4943-4952.
- Posey J E, Rosenfeld J A, James R A, et al. Molecular diagnostic experience of whole-exome sequencing in adult patients[J]. Genetics in Medicine, 2016, 18(7): 678-685.
- BoyantonJr.,B.L, Almradi,A, Espy,M.J. et al. Characterization of a novel melt curve by use of the Roche LightCycler HSV 1/2 analyte-specific reagent real-time PCR assay: Frequencies of this novel (low) melt curve and commonly encountered (intermediate) melt curves[J]. Journal of Clinical Microbiology, 2014, 52(3): 957-959.
- Lyon E, Wittwer C T. LightCycler technology in molecular diagnostics[J]. The Journal of Molecular Diagnostics, 2009, 11(2): 93-101.
- Molijn A, Kleter B, Quint W, et al. Molecular diagnosis of human papillomavirus (HPV) infections[J]. Journal of clinical virology, 2005, 32: 43-51.
- Hanein S, Perrault I, Gerber S, et al. Leber congenital amaurosis: comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype–phenotype correlations as a strategy for molecular diagnosis[J]. Human mutation, 2004, 23(4): 306-317.